 | |
 | |
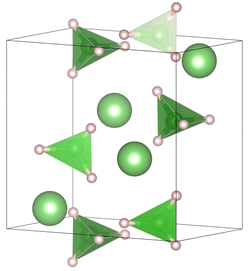 Unit cell of lithium borohydride at room temperature | |
| Names | |
|---|---|
| IUPAC name Lithium tetrahydridoborate(1–) | |
| Other names Lithium hydroborate, Lithium tetrahydroborate Borate(1-), tetrahydro-, lithium, lithium boranate | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.037.277 |
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| LiBH4 | |
| Molar mass | 21.784 g/mol |
| Appearance | White solid |
| Density | 0.666 g/cm3 [1] |
| Melting point | 268 °C (514 °F; 541 K) |
| Boiling point | 380 °C (716 °F; 653 K) decomposes |
| reacts | |
| Solubility in ether | 2.5 g/100 mL |
| Structure [2] | |
| orthorhombic | |
| Pnma | |
a = 7.17858(4), b = 4.43686(2), c = 6.80321(4) | |
Lattice volume (V) | 216.685(3) A3 |
Formula units (Z) | 4 |
| [4]B | |
| Thermochemistry | |
Heat capacity (C) | 82.6 J/(mol⋅K) |
Std molar entropy (S⦵298) | 75.7 J/(mol⋅K) |
Std enthalpy of formation (ΔfH⦵298) | −198.83 kJ/mol |
| Hazards | |
| >180 °C (356 °F; 453 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Lithium borohydride (LiBH4) is a borohydride and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages, being a stronger reducing agent and highly soluble in ethers, whilst remaining safer to handle than lithium aluminium hydride. [3]

