In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amino group ; or, equivalently, an acyl (alkanoyl) group joined to an amino group.

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a highly toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

Sodium amide, commonly called sodamide, is the inorganic compound with the formula NaNH2. It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Such impurities do not usually affect the utility of the reagent. NaNH2 conducts electricity in the fused state, its conductance being similar to that of NaOH in a similar state. NaNH2 has been widely employed as a strong base in organic synthesis.

Lithium hydride is an inorganic compound with the formula LiH. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a salt-like (ionic) hydride, it has a high melting point, and it is not soluble but reactive with all protic organic solvents. It is soluble and nonreactive with certain molten salts such as lithium fluoride, lithium borohydride, and sodium hydride. With a molar mass of 7.95 g/mol, it is the lightest ionic compound.

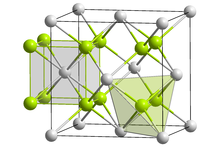
Lithium nitride is an inorganic compound with the chemical formula Li3N. It is the only stable alkali metal nitride. It is a reddish-pink solid with a high melting point.

Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred to by the same names. The anhydrous and hydrated forms are white crystalline solids.

Ammonia borane, also called borazane, is the chemical compound with the formula H3NBH3. The colourless or white solid is the simplest molecular boron-nitrogen-hydride compound. It has attracted attention as a source for hydrogen fuel, but is otherwise primarily of academic interest.

Lithium amide or lithium azanide is an inorganic compound with the chemical formula LiNH2. It is a white solid with a tetragonal crystal structure. Lithium amide can be made by treating lithium metal with liquid ammonia:

Aluminium hydride refers to a collection of inorganic compounds with the formula AlH3. As a gas, alane is a planar molecule. When generated in ether solutions, it exists as an ether adduct. Solutions of alane polymerizes to a solid, which exists in several crystallograhically distinguishable forms.

Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula LiN(Si(CH3)3)2. It is commonly abbreviated as LiHMDS or Li(HMDS) (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species.

Digallane is an inorganic compound with the chemical formula GaH2(H)2GaH2. It is the dimer of the monomeric compound gallane. The eventual preparation of the pure compound, reported in 1989, was hailed as a "tour de force." Digallane had been reported as early as 1941 by Wiberg; however, this claim could not be verified by later work by Greenwood and others. This compound is a colorless gas that decomposes above 0 °C.

Sodium aluminium hydride or sodium alanate is an inorganic compound with the chemical formula NaAlH4. It is a white pyrophoric solid that dissolves in tetrahydrofuran (THF), but not in diethyl ether or hydrocarbons. It has been evaluated as an agent for the reversible storage of hydrogen and it is used as a reagent for the chemical synthesis of organic compounds. Similar to lithium aluminium hydride, it is a salt consisting of separated sodium cations and tetrahedral AlH−
4 anions.
Transition metal hydrides are chemical compounds containing a transition metal bonded to hydrogen. Most transition metals form hydride complexes and some are significant in various catalytic and synthetic reactions. The term "hydride" is used loosely: some of them are acidic (e.g., H2Fe(CO)4), whereas some others are hydridic, having H−-like character (e.g., ZnH2).

Beryllium hydride is an inorganic compound with the chemical formula n. This alkaline earth hydride is a colourless solid that is insoluble in solvents that do not decompose it. Unlike the ionically bonded hydrides of the heavier Group 2 elements, beryllium hydride is covalently bonded.

Magnesium hydride is the chemical compound with the molecular formula MgH2. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.
Zinc hydride is an inorganic compound with the chemical formula ZnH2. It is a white, odourless solid which slowly decomposes into its elements at room temperature; despite this it is the most stable of the binary first row transition metal hydrides. A variety of coordination compounds containing Zn–H bonds are used as reducing agents, but ZnH2 itself has no common applications.

Metal amides (systematic name metal azanides) are a class of coordination compounds composed of a metal center with amide ligands of the form NR2−. Amido complexes of the parent amido ligand NH2− are rare compared to complexes with diorganylamido ligand, such as dimethylamido. Amide ligands have two electron pairs available for bonding.

William I. F. David is a professor of Materials Chemistry in the Department of Chemistry at the University of Oxford, an STFC Senior Fellow at the ISIS neutron source at the Rutherford Appleton Laboratory and a Fellow of St Catherine's College, Oxford.
The inorganic imide is an inorganic chemical compound containing