
Friedrich Wöhler FRS(For) HonFRSE was a German chemist known for his work in both organic and inorganic chemistry, being the first to isolate the chemical elements beryllium and yttrium in pure metallic form. He was the first to prepare several inorganic compounds, including silane and silicon nitride.
Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings.

Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as halides of carbon without carbon-hydrogen and carbon-carbon bonds, and certain compounds of carbon with nitrogen and oxygen.
In chemistry, a structural isomer of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept.
Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds—that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as inorganic chemistry.

Fulminic acid is an acid with the formula HCNO, more specifically H−C≡N+−O−. It is an isomer of isocyanic acid and of its elusive tautomer, cyanic acid, and also of isofulminic acid.

In chemistry, tautomers are structural isomers of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life.
In chemistry, a trimer is a molecule or polyatomic anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors often in competition with polymerization.
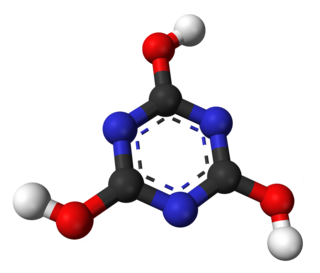
Cyanuric acid or 1,3,5-triazine-2,4,6-triol is a chemical compound with the formula (CNOH)3. Like many industrially useful chemicals, this triazine has many synonyms. This white, odorless solid finds use as a precursor or a component of bleaches, disinfectants, and herbicides. In 1997, worldwide production was 160 000 tonnes.

The cyanate ion is an anion with the chemical formula OCN−. It is a resonance of three forms: [O−−C≡N] (61%) ↔ [O=C=N−] (30%) ↔ [O+≡C−N2−] (4%).

CHNOPS and CHON are mnemonic acronyms for the most common elements in living organisms. "CHON" stands for carbon, hydrogen, oxygen, and nitrogen, which together make up more than 95 percent of the mass of biological systems. "CHNOPS" adds phosphorus and sulfur.

Potassium cyanate is an inorganic compound with the formula KOCN. It is a colourless solid. It is used to prepare many other compounds including useful herbicide. Worldwide production of the potassium and sodium salts was 20,000 tons in 2006.
The Wöhler synthesis is the conversion of ammonium cyanate into urea. This chemical reaction was described in 1828 by Friedrich Wöhler. It is often cited as the starting point of modern organic chemistry. Although the Wöhler reaction concerns the conversion of ammonium cyanate, this salt appears only as an (unstable) intermediate. Wöhler demonstrated the reaction in his original publication with different sets of reactants: a combination of cyanic acid and ammonia, a combination of silver cyanate and ammonium chloride, a combination of lead cyanate and ammonia and finally from a combination of mercury cyanate and cyanatic ammonia.
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.

In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism refers to the existence or possibility of isomers.
Sodium cyanate is the inorganic compound with the formula NaOCN. A white solid, it is the sodium salt of the cyanate anion.
![<span class="mw-page-title-main">Ammonium cyanate</span> Ionic chemical compound with formula [NH4]+ [OCN]-](https://upload.wikimedia.org/wikipedia/commons/thumb/d/dd/Ammonium-cyanate-3D-vdW.png/320px-Ammonium-cyanate-3D-vdW.png)
Ammoniumcyanate is an inorganic compound with the formula [NH4]+[OCN]−. It is a colorless, solid salt.

Sulfoxylic acid (H2SO2) (also known as hyposulfurous acid or sulfur dihydroxide) is an unstable oxoacid of sulfur in an intermediate oxidation state between hydrogen sulfide and dithionous acid. It consists of two hydroxy groups attached to a sulfur atom. Sulfoxylic acid contains sulfur in an oxidation state of +2. Sulfur monoxide (SO) can be considered as a theoretical anhydride for sulfoxylic acid, but it is not actually known to react with water.
The phosphaethynolate anion, also referred to as PCO, is the phosphorus-containing analogue of the cyanate anion with the chemical formula [PCO]− or [OCP]−. The anion has a linear geometry and is commonly isolated as a salt. When used as a ligand, the phosphaethynolate anion is ambidentate in nature meaning it forms complexes by coordinating via either the phosphorus or oxygen atoms. This versatile character of the anion has allowed it to be incorporated into many transition metal and actinide complexes but now the focus of the research around phosphaethynolate has turned to utilising the anion as a synthetic building block to organophosphanes.










![<span class="mw-page-title-main">Ammonium cyanate</span> Ionic chemical compound with formula [NH4]+ [OCN]-](https://upload.wikimedia.org/wikipedia/commons/thumb/d/dd/Ammonium-cyanate-3D-vdW.png/320px-Ammonium-cyanate-3D-vdW.png)

