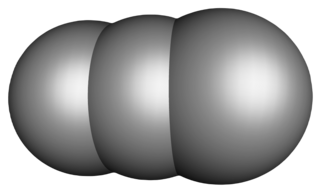In astronomy, extinction is the absorption and scattering of electromagnetic radiation by dust and gas between an emitting astronomical object and the observer. Interstellar extinction was first documented as such in 1930 by Robert Julius Trumpler. However, its effects had been noted in 1847 by Friedrich Georg Wilhelm von Struve, and its effect on the colors of stars had been observed by a number of individuals who did not connect it with the general presence of galactic dust. For stars that lie near the plane of the Milky Way and are within a few thousand parsecs of the Earth, extinction in the visual band of frequencies is roughly 1.8 magnitudes per kiloparsec.

In astronomy, Bok globules are isolated and relatively small dark nebulae, containing dense cosmic dust and gas from which star formation may take place. Bok globules are found within H II regions, and typically have a mass of about 2 to 50 solar masses contained within a region about a light year or so across (about 4.5×1047 m3). They contain molecular hydrogen (H2), carbon oxides and helium, and around 1% (by mass) silicate dust. Bok globules most commonly result in the formation of double- or multiple-star systems.

The Caltech Submillimeter Observatory (CSO) was a 10.4-meter (34 ft) diameter submillimeter wavelength telescope situated alongside the 15-meter (49 ft) James Clerk Maxwell Telescope (JCMT) at Mauna Kea Observatories. It was engaged in submillimeter astronomy, of the terahertz radiation band. The telescope closed on September 18, 2015. As of April 2019, the telescope is set to be dismantled and its site remediated in the near future as part of the Mauna Kea Comprehensive Management Plan.

The trihydrogen cation or protonated molecular hydrogen is a cation with formula H+
3, consisting of three hydrogen nuclei (protons) sharing two electrons.
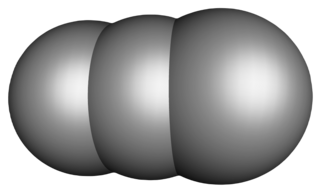
Tricarbon is an inorganic compound with the chemical formula C
2(μ-C). It is a colourless gas that only persists in dilution or solution as an adduct. It is one of the simplest unsaturated carbenes. Tricarbon can be found in interstellar space and can be produced in the laboratory by a process called laser ablation.

CW Leonis or IRC +10216 is a variable carbon star that is embedded in a thick dust envelope. It was first discovered in 1969 by a group of astronomers led by Eric Becklin, based upon infrared observations made with the 62 inches (1.6 m) Caltech Infrared Telescope at Mount Wilson Observatory. Its energy is emitted mostly at infrared wavelengths. At a wavelength of 5 μm, it was found to have the highest flux of any object outside the Solar System.
Octatetraynyl radical is an organic free radical with eight carbon atoms linked in a linear chain with alternating single bonds and triple bonds.

Carbon monosulfide is a chemical compound with the formula CS. This diatomic molecule is the sulfur analogue of carbon monoxide, and is unstable as a solid or a liquid, but it has been observed as a gas both in the laboratory and in the interstellar medium. The molecule resembles carbon monoxide with a triple bond between carbon and sulfur. The molecule is not intrinsically unstable, but it tends to polymerize. This tendency reflects the greater stability of C–S single bonds.
Sagittarius B2 is a giant molecular cloud of gas and dust that is located about 120 parsecs (390 ly) from the center of the Milky Way. This complex is the largest molecular cloud in the vicinity of the core and one of the largest in the galaxy, spanning a region about 45 parsecs (150 ly) across. The total mass of Sgr B2 is about 3 million times the mass of the Sun. The mean hydrogen density within the cloud is 3000 atoms per cm3, which is about 20–40 times denser than a typical molecular cloud.
Propynylidyne is a chemical compound that has been identified in interstellar space.

HCNH+, also known as protonated hydrogen cyanide, is a molecular ion of astrophysical interest. It also exists in the condensed state when formed by superacids.

The cyano radical (or cyanido radical) is a radical with molecular formula CN, sometimes written •CN. The cyano radical was one of the first detected molecules in the interstellar medium, in 1938. Its detection and analysis was influential in astrochemistry. The discovery was confirmed with a coudé spectrograph, which was made famous and credible due to this detection. ·CN has been observed in both diffuse clouds and dense clouds. Usually, CN is detected in regions with hydrogen cyanide, hydrogen isocyanide, and HCNH+, since it is involved in the creation and destruction of these species (see also Cyanogen).

Imidogen is an inorganic compound with the chemical formula NH. Like other simple radicals, it is highly reactive and consequently short-lived except as a dilute gas. Its behavior depends on its spin multiplicity.
Interstellar ice consists of grains of volatiles in the ice phase that form in the interstellar medium. Ice and dust grains form the primary material out of which the Solar System was formed. Grains of ice are found in the dense regions of molecular clouds, where new stars are formed. Temperatures in these regions can be as low as 10 K, allowing molecules that collide with grains to form an icy mantle. Thereafter, atoms undergo thermal motion across the surface, eventually forming bonds with other atoms. This results in the formation of water and methanol. Indeed, the ices are dominated by water and methanol, as well as ammonia, carbon monoxide and carbon dioxide. Frozen formaldehyde and molecular hydrogen may also be present. Found in lower abundances are nitriles, ketones, esters and carbonyl sulfide. The mantles of interstellar ice grains are generally amorphous, becoming crystalline only in the presence of a star.
CO-0.40-0.22 is a high velocity compact gas cloud near the centre of the Milky Way. It is 200 light years away from the centre in the central molecular zone. The cloud is in the shape of ellipse. The differences in the velocity, termed velocity dispersion, of the gas is unusually high at 100 km/s.

Tricarbon monoxide C3O is a reactive radical oxocarbon molecule found in space, and which can be made as a transient substance in the laboratory. It can be trapped in an inert gas matrix or made as a short lived gas. C3O can be classified as a ketene or an oxocumulene a kind of heterocumulene.
A heterocumulene is a molecule or ion containing a chain of at least three double bonds between consecutive atoms, in which one or more atoms in the doubly bonded chain is a heteroatom. Such species are analogous to a cumulene in which the chain of doubly bonded atoms contains only carbon, except that at least one carbon is replaced by a heteroatom. Some authors relax the definition to include species with chains of only two double bonds between consecutive atoms, also known as heteroallenes.

Thioxoethenylidene, is a reactive heteroallene molecule with formula CCS.

DL Tauri is a young T Tauri-type pre-main sequence stars in the constellation of Taurus about 522 light years away, belonging to the Taurus Molecular Cloud. It is partially obscured by the foreground gas cloud rich in carbon monoxide, and is still accreting mass, producing 0.14 L☉ due to release of accretion energy. The stellar spectrum shows the lines of ionized oxygen, nitrogen, sulfur and iron.