| |||
 | |||
 | |||
| Names | |||
|---|---|---|---|
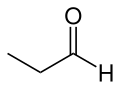
| IUPAC name Propionaldehyde | |||
| Preferred IUPAC name Propanal | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.204 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1275 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C3H6O | |||
| Molar mass | 58.080 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Pungent and fruity | ||
| Density | 0.81 g cm−3 | ||
| Melting point | −81 °C (−114 °F; 192 K) | ||
| Boiling point | 46 to 50 °C (115 to 122 °F; 319 to 323 K) | ||
| 20 g/100 mL | |||
| −34.32·10−6 cm3/mol | |||
| Viscosity | 0.6 cP at 20 °C | ||
| Structure | |||
| C1, O: sp2 C2, C3: sp3 | |||
| 2.52 D | |||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Danger | |||
| H225, H302, H315, H318, H332, H335 [1] | |||
| P210, P261, P280, P304+P340+P312, P305+P351+P338, P310, P403+P235 [1] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −26 °C (−15 °F; 247 K) | ||
| 175 °C (347 °F; 448 K) | |||
| Related compounds | |||
Related aldehydes | Acetaldehyde Butyraldehyde | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a pungent and fruity odour. It is produced on a large scale industrially.