See also
- Propanal (propionaldehyde) differs in spelling from propanol by a single letter and is a different compound.
- Propranolol is a drug used for reducing blood pressure and hand tremors.
There are two isomers of propanol.

In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of hydrocarbons, conferring hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur.

In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.

Hexafluoroisopropanol, commonly abbreviated HFIP, is the organic compound with the formula (CF3)2CHOH. This fluoroalcohol finds use as solvent in organic chemistry. Hexafluoro-2-propanol is transparent to UV light with high density, low viscosity and low refractive index. It is a colorless, volatile liquid with a pungent odor.
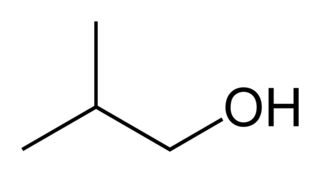
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as i-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers are 1-butanol, 2-butanol, and tert-butanol, all of which are important industrially.

Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.

Propyl acetate, also known as propyl ethanoate, is an organic compound. Nearly 20,000 tons are produced annually for use as a solvent. This colorless liquid is known by its characteristic odor of pears. Due to this fact, it is commonly used in fragrances and as a flavor additive. It is formed by the esterification of acetic acid and propan-1-ol, often via Fischer–Speier esterification, with sulfuric acid as a catalyst and water produced as a byproduct.

In chemistry, the heteropolymetalates are a subset of the polyoxometalates, which consist of three or more transition metal oxyanions linked together by shared oxygen atoms to form a closed 3-dimensional molecular framework. In contrast to isopolymetalates, which contain only one kind of metal atom, the heteropolymetalates contain differing main group oxyanions. The metal atoms are usually group 6 or less commonly group 5 transition metals in their highest oxidation states. They are usually colorless to orange, diamagnetic anions. For most heteropolymetalates the W, Mo, or V, is complemented by main group oxyanions phosphate and silicate. Many exceptions to these general statements exist, and the class of compounds includes hundreds of examples.

1-Propanol is a primary alcohol with the formula CH3CH2CH2OH and sometimes represented as PrOH or n-PrOH. It is a colourless liquid and an isomer of 2-propanol. 1-Propanol is used as a solvent in the pharmaceutical industry, mainly for resins and cellulose esters, and, sometimes, as a disinfecting agent.
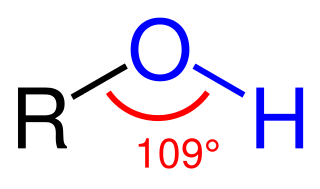
The suffix –ol is used in organic chemistry principally to form names of organic compounds containing the hydroxyl (–OH) group, mainly alcohols. The suffix was extracted from the word alcohol.

1-Aminopropan-2-ol is the organic compound with the formula CH3CH(OH)CH2NH2. It is an amino alcohol. The term isopropanolamine may also refer more generally to the additional homologs diisopropanolamine (DIPA) and triisopropanolamine (TIPA).

In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Diamond and graphite are a familiar example; they are isomers of carbon. Isomerism refers to the existence or possibility of isomers.
Isopropyl alcohol is a colorless, flammable organic compound with a pungent alcoholic odor.
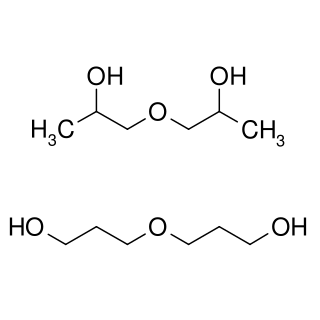
Dipropylene glycol is a mixture of three isomeric chemical compounds, 4-oxa-2,6-heptandiol, 2-(2-hydroxy-propoxy)-propan-1-ol, and 2-(2-hydroxy-1-methyl-ethoxy)-propan-1-ol. It is a colorless, nearly odorless liquid with a high boiling point and low toxicity.
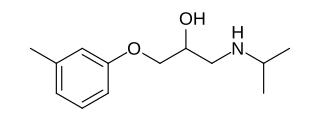
Toliprolol is a beta adrenergic receptor antagonist.
Chloropropanols are chlorohydrins related to propanols containing chloride functional group. Eight isomers are possible. Two of these derivatives, 1,3-dichloropropanol (1,3-DCP) and 3-chloropropane-1,2-diol (3-MCPD), are carcinogenic contaminants in processed foods. Several isomers are encountered in industrial chemistry.
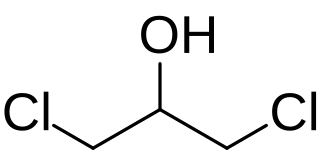
1,3-Dichloropropan-2-ol (1,3-DCP) is an organic compound with the formula HOCH2CHClCH2Cl. It is a colorless liquid. It is an intermediate in the production of epichlorohydrin.
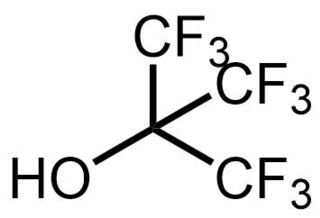
Nonafluoro-tert-butyl alcohol is a fluoroalcohol. It is the perfluorinated analog of tert-butyl alcohol. Notably, as a consequence of its electron withdrawing fluorine substituents, it is very acidic for an alcohol, with a pKa value of 5.4, similar to that of a carboxylic acid. As another consequence of being a perfluorinated compound, it is also one of the lowest boiling alcohols, with a boiling point lower than that of methanol.
In organic chemistry, propanolamine can describe any of the following parent compounds:

MXiPr is a recreational designer drug with dissociative effects. It is an arylcyclohexylamine derivative, related to drugs such as ketamine and methoxetamine. It was first identified in Slovenia in December 2020, and was made illegal in Hungary in April 2021.
Methionol is a methyl sulfide derived from propan-1-ol. It is found in nature, including as a metabolite of yeast and bacillus anthracis. It is a sulphurous aroma component of many foods, such as wine, cheese and roasted coffee. It is classed as an irritant. It has a very low olfactory threshold.