| |
 | |
| Names | |
|---|---|
| Pronunciation | /ˈprəʊpən.wən.ɒl/ |
| Preferred IUPAC name Propan-1-ol [1] | |
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| 1098242 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.679 |
| EC Number |
|
| 25616 | |
| KEGG | |
| MeSH | 1-Propanol |
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1274 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C3H8O | |
| Molar mass | 60.096 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | mild, alcohol-like [2] |
| Density | 0.803 g/mL |
| Melting point | −126 °C; −195 °F; 147 K |
| Boiling point | 97 to 98 °C; 206 to 208 °F; 370 to 371 K |
| miscible | |
| log P | 0.329 |
| Vapor pressure | 1.99 kPa (at 20 °C) |
| Acidity (pKa) | 16 |
| Basicity (pKb) | −2 |
| −45.176·10−6 cm3/mol | |
Refractive index (nD) | 1.387 |
| Viscosity | 1.959 mPa·s (at 25 °C) [3] |
| 1.68 D | |
| Thermochemistry | |
Heat capacity (C) | 143.96 J/(K·mol) |
Std molar entropy (S⦵298) | 192.8 J/(K·mol) |
Std enthalpy of formation (ΔfH⦵298) | −302.79…−302.29 kJ/mol |
Std enthalpy of combustion (ΔcH⦵298) | −2.02156…−2.02106 MJ/mol |
| Pharmacology | |
| D08AX03 ( WHO ) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Flammable liquid |
| GHS labelling: | |
  | |
| Danger | |
| H225, H302, H318, H336 | |
| P210, P261, P280, P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 22 °C (72 °F; 295 K) |
| 371 °C (700 °F; 644 K) | |
| Explosive limits | 2.2–13.7% [2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 2800 mg/kg (rabbit, oral) 1699 mg/kg (mouse, oral) 1870 mg/kg (rat, oral) [4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | TWA 200 ppm (500 mg/m3) [2] |
REL (Recommended) | TWA 200 ppm (500 mg/m3) ST 250 ppm (625 mg/m3) [skin] [2] |
IDLH (Immediate danger) | 800 ppm [2] |
| Related compounds | |
Related compounds | Propane Isopropyl alcohol Propanamine Ethanol Butanol |
| Supplementary data page | |
| 1-Propanol (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
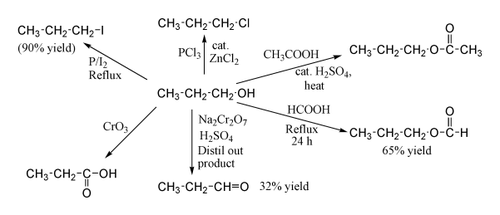
1-Propanol (also propan-1-ol, propanol, n-propyl alcohol) is a primary alcohol with the formula CH3CH2CH2OH and sometimes represented as PrOH or n-PrOH. It is a colourless liquid and an isomer of 2-propanol. 1-Propanol is used as a solvent in the pharmaceutical industry, mainly for resins and cellulose esters, and, sometimes, as a disinfecting agent.