
Sex hormones, also known as sex steroids, gonadocorticoids and gonadal steroids, are steroid hormones that interact with vertebrate steroid hormone receptors. The sex hormones include the androgens, estrogens, and progestogens. Their effects are mediated by slow genomic mechanisms through nuclear receptors as well as by fast nongenomic mechanisms through membrane-associated receptors and signaling cascades. The polypeptide hormones luteinizing hormone, follicle-stimulating hormone and gonadotropin-releasing hormone are usually not regarded as sex hormones, although they play major sex-related roles.

Homosalate is an organic compound used in some sunscreens. It is made by the Fischer–Speier esterification of salicylic acid and 3,3,5-trimethylcyclohexanol, the latter being a hydrogenated derivative of isophorone. Contained in 45% of U.S. sunscreens, it is used as a chemical UV filter. The salicylic acid portion of the molecule absorbs ultraviolet rays with a wavelength from 295 nm to 315 nm, protecting the skin from sun damage. The hydrophobic trimethyl cyclohexane functional group provides greasiness that prevents it from dissolving in water.

Etynodiol, or ethynodiol, is a steroidal progestin of the 19-nortestosterone group which was never marketed. A diacylated derivative, etynodiol diacetate, is used as a hormonal contraceptive. Etynodiol is sometimes used as a synonym for etynodiol diacetate.

Chlorotrianisene (CTA), also known as tri-p-anisylchloroethylene (TACE) and sold under the brand name Tace among others, is a nonsteroidal estrogen related to diethylstilbestrol (DES) which was previously used in the treatment of menopausal symptoms and estrogen deficiency in women and prostate cancer in men, among other indications, but has since been discontinued and is now no longer available. It is taken by mouth.

Ormeloxifene, also known as centchroman, is one of the selective estrogen receptor modulators, or SERMs, a class of medication which acts on the estrogen receptor. It is best known as a nonsteroidal oral contraceptive which is taken once per week. In India, ormeloxifene has been available as birth control since the early 1990s, and it was marketed there under the trade name Saheli, currently available free-of-cost for the women in India as Chhaya (Centchroman). Ormeloxifene has also been licensed under the trade names Novex-DS, Centron, and Sevista.
Hormone replacement therapy (HRT), also known as menopausal hormone therapy or postmenopausal hormone therapy, is a form of hormone therapy used to treat symptoms associated with female menopause. These symptoms can include hot flashes, vaginal atrophy, accelerated skin aging, vaginal dryness, decreased muscle mass, sexual dysfunction, and bone loss. They are in large part related to the diminished levels of sex hormones that occur during menopause.

Triphenylethylene (TPE) is a simple aromatic hydrocarbon that possesses weak estrogenic activity. Its estrogenic effects were discovered in 1937. TPE was derived from structural modification of the more potent estrogen diethylstilbestrol, which is a member of the stilbestrol group of nonsteroidal estrogens.

Doisynolic acid is a synthetic, nonsteroidal, orally active estrogen that was never marketed. The reaction of estradiol or estrone with potassium hydroxide, a strong base, results in doisynolic acid as a degradation product, which retains high estrogenic activity, and this reaction was how the drug was discovered, in the late 1930s. The drug is a highly active and potent estrogen by the oral or subcutaneous route. The reaction of equilenin or dihydroequilenin with potassium hydroxide was also found to produce bisdehydrodoisynolic acid, the levorotatory isomer of which is an estrogen with an "astonishingly" high degree of potency, while the dextrorotatory isomer is inactive. Doisynolic acid was named after Edward Adelbert Doisy, a pioneer in the field of estrogen research and one of the discoverers of estrone.
Estradiol palmitate, or estradiol monopalmitate, also known as estradiol 17β-hexadecanoate, is a naturally occurring steroidal estrogen and an estrogen ester – specifically, the C17β palmitate ester of estradiol. It occurs in the body as a very long-lasting metabolite and prohormone of estradiol. The compound has no affinity for the estrogen receptor, requiring transformation into estradiol for its estrogenic activity. In addition to its endogenous role, estradiol palmitate was formerly used as a fattening agent in chickens under the brand name Esmopal.

17α-Dihydroequilin, or α-dihydroequilin, also known as 7-dehydro-17α-estradiol, as well as estra-1,3,5(10),7-tetraene-3,17α-diol, is a naturally occurring steroidal estrogen found in horses which is closely related to equilin, equilenin, and 17α-estradiol. The compound, as the 3-sulfate ester sodium salt, is present in conjugated estrogens (Premarin), a pharmaceutical extract of the urine of pregnant mares, and is the third highest quantity constituent in the formulation (13.8%). The compound has been studied clinically.

Mytatrienediol, also known as 16α-methyl-16β-epiestriol 3-methyl ether or 16β-hydroxy-16α-methylestradiol 3-methyl ether, is a synthetic steroidal estrogen medication and an estrogen ether which was derived from estriol and was developed for clinical use in the late 1950s but was never marketed. It was investigated as a weak and mildly estrogenic medication for men to treat atherosclerosis, improve serum lipid profiles, and reduce the risk of myocardial infarction. However, while preclinical research supported the profile of mytatriendiol as a weak estrogen, the medication was found in clinical trials to produce estrogenic side effects including feminization, breast pain, and gynecomastia in men similarly and comparably to other estrogens such as ethinylestradiol and conjugated estrogens, and its side effects ultimately precluded its use. The medication was also studied to treat bone pain in patients with multiple myeloma, metastatic bone disease, and osteoporosis, with effectiveness seen.

Dianethole is a naturally occurring organic compound that is found in anise and fennel. It is a dimeric polymer of anethole. It has estrogenic activity, and along with anethole and photoanethole, may be responsible for the estrogenic effects of anise and fennel. These compounds bear resemblance to the estrogens stilbene and diethylstilbestrol, which may explain their estrogenic activity. In fact, it is said that diethylstilbestrol and related drugs were originally modeled after dianethole and photoanethole.

Bisdehydrodoisynolic acid (BDDA), as the (Z)-isomer ( -BDDA), is a synthetic, nonsteroidal estrogen related to doisynolic acid that was never marketed. It is one of the most potent estrogens known, although it has more recently been characterized as a selective estrogen receptor modulator (SERM). BDDA and other doisynolic acid derivatives display relatively low affinity accompanied by disproportionately high estrogenic potency in vivo, which was eventually determined to be due to transformation into metabolites with greater estrogenic activity. The drug was discovered in 1947 as a degradation product of the reaction of equilenin or dihydroequilenin with potassium hydroxide. It is the seco-analogue of equilenin, while doisynolic acid is the seco-analogue of estrone. These compounds, along with diethylstilbestrol, can be considered to be open-ring analogues of estradiol. The methyl ether of BDDA, doisynoestrol, is also an estrogen, and in contrast to BDDA, has been marketed.

Allenolic acid, or allenoic acid, is a synthetic, nonsteroidal estrogen discovered in 1947 or 1948 that, although studied clinically, was never marketed. It is an open-ring or seco-analogue of steroidal estrogens like estrone and equilenin. The compound was named after Dr. Edgar Allen, one of the pioneers in estrogen research. Although described as an estrogen, allenolic acid probably is totally inactive at the receptor, whereas a derivative, allenestrol, is reported to be a potent estrogen. Another derivative of allenolic acid, methallenestril, is also a potent estrogen and, in contrast to allenolic acid and allenestrol, has been marketed.
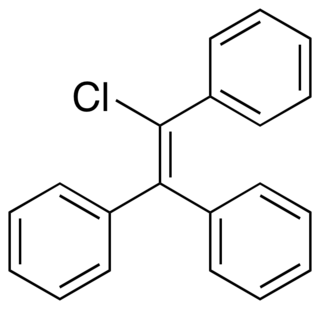
Triphenylchloroethylene, or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.

Photoanethole is a naturally occurring organic compound that is found in anise and fennel. It has estrogenic activity, and along with anethole and dianethole, may be responsible for the estrogenic effects of anise and fennel. These compounds bear resemblance to the estrogens stilbene and diethylstilbestrol, which may explain their estrogenic activity. In fact, it is said that diethylstilbestrol and related drugs were originally modeled after photoanethole and dianethole.
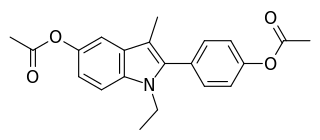
Zindoxifene is a nonsteroidal selective estrogen receptor modulator (SERM) that was under development in the 1980s and early 1990s for the treatment of breast cancer but was not marketed. It showed estrogenic-like activity in preclinical studies and failed to demonstrate effectiveness as a treatment for breast cancer in clinical trials. Zindoxifene was the lead compound of the distinct 2-phenylindole class of SERMs, and the marketed SERM bazedoxifene was derived from the major active metabolite of zindoxifene, D-15414. Zindoxifene was first described in 1984.

Hippulin, also known as Δ8-14-isoestrone, as well as 14-isoestra-1,3,5(10),8-tetraen-3-ol-17-one, is a naturally occurring estrogen found in horses and an isomer of equilin. The compound, likely in sodium sulfate form, is a component of conjugated estrogens (Premarin), a pharmaceutical extract of the urine of pregnant mares, though it is present only in small amounts in pregnant mare urine. It has been reported by possess either equivalent estrogenic activity to that of equilin or only slight estrogenic activity. The compound was first described in 1932.

Estrone sulfate (E1S) is an estrogen medication and naturally occurring steroid hormone. It is used in menopausal hormone therapy among other indications. As the sodium salt, it is the major estrogen component of conjugated estrogens (Premarin) and esterified estrogens. In addition, E1S is used on its own as the piperazine salt estropipate. The compound also occurs as a major and important metabolite of estradiol and estrone. E1S is most commonly taken by mouth, but in the form of Premarin can also be taken by parenteral routes such as transdermal, vaginal, and injection.

NC 45-0095 is a synthetic nonsteroidal selective estrogen receptor modulator (SERM) which was under development by Novo Nordisk for the treatment of postmenopausal osteoporosis but was never marketed. It is a partial agonist of the estrogen receptor (IC50 (for binding inhibition) = 9.5 nM; EC50 = 13 nM) with mixed estrogenic and antiestrogenic activity, and shows full estrogenic activity in bone and uterus (Emax (relative to moxestrol, in Ishikawa endometrial cancer cell line) = 105%). The compound is a pyrroloindolizine derivative. Its development was discontinued by 2003.


















