
Ludwig Karl Martin Leonhard Albrecht Kossel was a German biochemist and pioneer in the study of genetics. He was awarded the Nobel Prize for Physiology or Medicine in 1910 for his work in determining the chemical composition of nucleic acids, the genetic substance of biological cells.

Radium chloride (RaCl2) is a salt of radium and chlorine, and the first radium compound isolated in a pure state. Marie Curie and André-Louis Debierne used it in their original separation of radium from barium. The first preparation of radium metal was by the electrolysis of a solution of this salt using a mercury cathode.

Adolf Friedrich Johann Butenandt was a German biochemist. He was awarded the Nobel Prize in Chemistry in 1939 for his "work on sex hormones." He initially rejected the award in accordance with government policy, but accepted it in 1949 after World War II. He was President of the Max Planck Society from 1960 to 1972. He was also the first, in 1959, to discover the structure of the sex pheromone of silkworms which he named as bombykol.
Paraldehyde is the cyclic trimer of acetaldehyde molecules. Formally, it is a derivative of 1,3,5-trioxane, with a methyl group substituted for a hydrogen atom at each carbon. The corresponding tetramer is metaldehyde. A colourless liquid, it is sparingly soluble in water and highly soluble in ethanol. Paraldehyde slowly oxidizes in air, turning brown and producing an odour of acetic acid. It quickly reacts with most plastics and rubber.
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid itself is not pharmacologically active. The compound was first synthesised by Adolf von Baeyer.

Dichlorine monoxide is an inorganic compound with the molecular formula Cl2O. It was first synthesised in 1834 by Antoine Jérôme Balard, who along with Gay-Lussac also determined its composition. In older literature it is often referred to as chlorine monoxide, which can be a source of confusion as that name now refers to the neutral species ClO.
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.
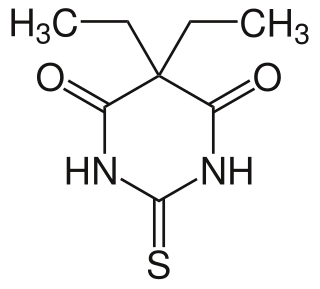
Thiobarbital is a drug which is a barbiturate derivative. It is the thiobarbiturate analogue of barbital.
Fructosephosphates are sugar phosphates based upon fructose, and are common in the biochemistry of cells.

Indium(III) bromide, (indium tribromide), InBr3, is a chemical compound of indium and bromine. It is a Lewis acid and has been used in organic synthesis.
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.

Fenozolone (Ordinator) was developed by Laboratoires Dausse in the 1960s and is a psychostimulant related to pemoline.

The Zeitschrift für Naturforschung B: A Journal of Chemical Sciences is a monthly peer-reviewed scientific journal. The journal publishes "fundamental studies in all areas of inorganic chemistry, organic chemistry, and analytical chemistry" in both English and German. Articles in German are required to be accompanied by an English-language title and abstract. According to the Journal Citation Reports, the journal has a 2014 impact factor of 0.744. The editors-in-chief are Gerhard Müller and Annette Schier.
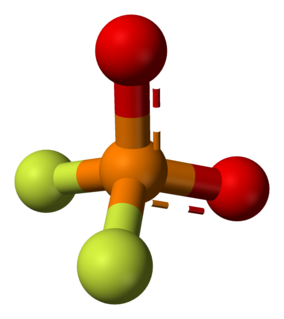
Difluorophosphate or difluorodioxophosphate or phosphorodifluoridate is an anion with formula PO
2F−
2. It has a single negative charge and resembles perchlorate (ClO−
4) and monofluorosulfonate (SO3F−) in shape and compounds. These ions are isoelectronic, along with tetrafluoroaluminate, phosphate, orthosilicate, and sulfate. It forms a series of compounds. The ion is toxic to mammals as it causes blockage to iodine uptake in the thyroid. However it is degraded in the body over several hours.
Wilhelm Karl Klemm was an inorganic and physical chemist. Klemm did extensive work on intermetallic compounds, rare earth metals, transition elements and compounds involving oxygen and fluorine. He and Heinrich Bommer were the first to isolate elemental erbium (1934) and ytterbium (1936). Klemm refined Eduard Zintl's ideas about the structure of intermetallic compounds and their connections to develop the Zintl-Klemm concept.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.
Ernst Klenk was a German biochemist, known as a pioneer in research on biolipids, their metabolism, and diseases caused by biolipid disorders.
Carbide chlorides are mixed anion compounds containing chloride anions and anions consisting entirely of carbon. In these compounds there is no bond between chlorine and carbon. But there is a bond between a metal and carbon. Many of these compounds are cluster compounds, in which metal atoms encase a carbon core, with chlorine atoms surrounding the cluster. The chlorine may be shared between clusters to form polymers or layers. Most carbon chloride compounds contain rare earth elements. Some are known from group 4 elements. The hexatungsten carbon cluster can be oxidised and reduced, and so have different numbers of chlorine atoms included.
Carbide bromides are mixed anion compounds containing bromide and carbide anions. Many carbide bromides are cluster compounds, containing on, two or more carbon atoms in a core, surrounded by a layer of metal atoms, encased in a shell of bromide ions. These ions may be shared between clusters to form chains, double chains or layers.
Carbide iodides are mixed anion compounds containing iodide and carbide anions. Many carbide iodides are cluster compounds, containing one, two or more carbon atoms in a core, surrounded by a layer of metal atoms, encased in a shell of iodide ions. These ions may be shared between clusters to form chains, double chains or layers.










