
Metenolone, or methenolone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as metenolone acetate and metenolone enanthate. Metenolone esters are used mainly in the treatment of anemia due to bone marrow failure. Metenolone acetate is taken by mouth, while metenolone enanthate is given by injection into muscle.
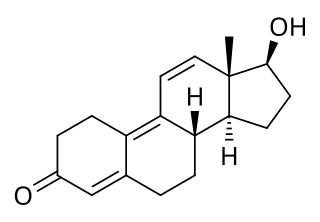
Trenbolone is an androgen and anabolic steroid (AAS) of the nandrolone group which itself was never marketed. Trenbolone ester prodrugs, including trenbolone acetate and trenbolone hexahydrobenzylcarbonate, are or have been marketed for veterinary and clinical use. Trenbolone acetate is used in veterinary medicine in livestock to increase muscle growth and appetite, while trenbolone hexahydrobenzylcarbonate was formerly used clinically in humans but is now no longer marketed. In addition, although it is not approved for clinical or veterinary use, trenbolone enanthate is sometimes sold on the black market under the nickname Trenabol.

Quinbolone, sold under the brand names Anabolicum and Anabolvis, is an androgen and anabolic steroid (AAS) which was previously marketed in Italy. It was developed by Parke-Davis as a viable orally-administered AAS with little or no liver toxicity.
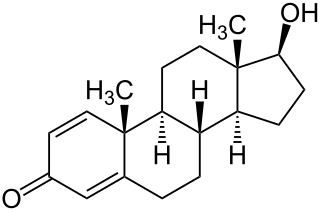
Boldenone, is a naturally occurring anabolic–androgenic steroid (AAS) and the 1(2)-dehydrogenated analogue of testosterone. Boldenone itself has never been marketed; as a pharmaceutical drug, it is used as boldenone undecylenate, the undecylenate ester.
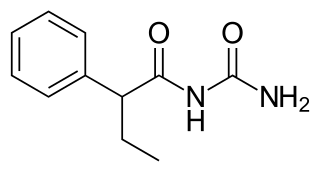
Pheneturide, also known as phenylethylacetylurea, is an anticonvulsant of the ureide class. Conceptually, it can be formed in the body as a metabolic degradation product from phenobarbital. It is considered to be obsolete and is now seldom used. It is marketed in Europe, including in Poland, Spain and the United Kingdom. Pheneturide has a similar profile of anticonvulsant activity and toxicity relative to phenacemide. As such, it is only used in cases of severe epilepsy when other, less-toxic drugs have failed. Pheneturide inhibits the metabolism and thus increases the levels of other anticonvulsants, such as phenytoin.

Lynestrenol, sold under the brand names Exluton and Ministat among others, is a progestin medication which is used in birth control pills and in the treatment of gynecological disorders. The medication is available both alone and in combination with an estrogen. It is taken by mouth.

Niaprazine (INN) is a sedative-hypnotic drug of the phenylpiperazine group. It has been used in the treatment of sleep disturbances since the early 1970s in several European countries including France, Italy, and Luxembourg. It is commonly used with children and adolescents on account of its favorable safety and tolerability profile and lack of abuse potential.

Corbadrine, also known as levonordefrin (USAN) and α-methylnorepinephrine, is a catecholamine sympathomimetic used as a topical nasal decongestant and vasoconstrictor in dentistry in the United States,.

Maraviroc, sold under the brand names Selzentry (US) and Celsentri (EU), is an antiretroviral medication used to treat HIV infection. It is taken by mouth. It is in the CCR5 receptor antagonist class.
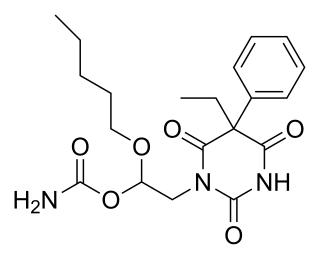
Febarbamate, also known as phenobamate, is an anxiolytic and tranquilizer of the barbiturate and carbamate families which is used in Europe by itself and as part of a combination drug formulation called tetrabamate.

Mestanolone, also known as methylandrostanolone and sold under the brand names Androstalone and Ermalone among others, is an androgen and anabolic steroid (AAS) medication which is mostly no longer used. It is still available for use in Japan however. It is taken by mouth.

Norfenefrine is an adrenergic agent used as a sympathomimetic drug which is marketed in Europe, Japan, and Mexico. Along with its structural isomer p-octopamine and the tyramines, norfenefrine is a naturally occurring, endogenous trace amine and plays a role as a minor neurotransmitter in the brain.

Medifoxamine, previously sold under the brand names Clédial and Gerdaxyl, is an atypical antidepressant with additional anxiolytic properties acting via dopaminergic and serotonergic mechanisms which was formerly marketed in France and Spain, as well as Morocco. The drug was first introduced in France sometime around 1990. It was withdrawn from the market in 1999 (Morocco) and 2000 (France) following incidences of hepatotoxicity.

Retigabine (INN) or ezogabine (USAN) is an anticonvulsant used as an adjunctive treatment for partial epilepsies in treatment-experienced adult patients. The drug was developed by Valeant Pharmaceuticals and GlaxoSmithKline. It was approved by the European Medicines Agency under the trade name Trobalt on March 28, 2011, and by the United States Food and Drug Administration (FDA), under the trade name Potiga, on June 10, 2011. Production was discontinued in June 2017.

Promegestone, sold under the brand name Surgestone, is a progestin medication which is used in menopausal hormone therapy and in the treatment of gynecological disorders. It is taken by mouth.

PCPr is an arylcyclohexylamine dissociative anesthetic drug with hallucinogenic and stimulant effects. It is around the same potency as phencyclidine, although slightly less potent than its ethyl homologue eticyclidine, and has reportedly been sold as a designer drug in Germany and other European countries since the late 1990s.
Tetrabamate is a combination drug formulation of febarbamate, difebarbamate, and phenobarbital which was marketed in France and Spain and was used to treat anxiety and alcohol withdrawal-associated muscle tremors, agitation, and depression. It was largely, but not completely discontinued on April 4, 1997 after over 30 years of use due to reports of hepatitis and acute liver failure. The decision to restrict the use of the drug had been long-awaited.

Alectinib is an oral drug that blocks the activity of anaplastic lymphoma kinase (ALK) and is used to treat non-small-cell lung cancer (NSCLC). It was developed by Chugai Pharmaceutical Co. Japan, which is part of the Hoffmann-La Roche group.
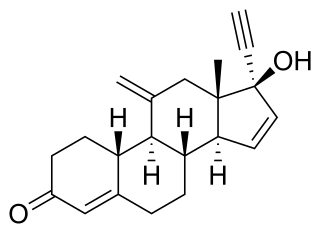
Tosagestin, also known as 11-methylene-δ15-norethisterone or 17α-ethynyl-11-methylene-19-nor-δ15-testosterone, is a progestin of the 19-nortestosterone group which was under development by Organon in the United States and Europe as a hormonal contraceptive and for the treatment of menopausal symptoms but was never marketed.

Vibegron, sold under the brand name Gemtesa, is a medication for the treatment of overactive bladder.



















