 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Alloferin |
| Other names | Ro 4-3816, diallylnortoxiferine |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | not metabolized |
| Elimination half-life | 2–4 hours |
| Excretion | 70–90% unchanged in urine 1.3 mL/kg/min |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.035.648 |
| Chemical and physical data | |
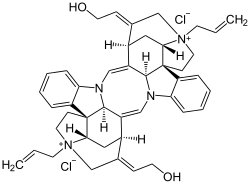
| Formula | C44H50N4O2+2 |
| Molar mass | 666.910 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Alcuronium chloride (formerly marketed as Alloferin) is a neuromuscular blocking (NMB) agent, alternatively referred to as a skeletal muscle relaxant. It is a semi-synthetic substance prepared from C-toxiferine I, [1] a bis-quaternary alkaloid obtained from Strychnos toxifera . C-toxiferine I itself has been tested for its pharmacological action and noted to be a very long acting neuromuscular blocking agent [2] For a formal definition of the durations of actions associated with NMB agents, see page for gantacurium. The replacement of both the N-methyl groups with N-allyl moieties yielded N,N-diallyl-bis-nortoxiferine, now recognized as alcuronium.
Contents
Inclusion of the allylic functions presented an enhanced potential area of biotransformation, and thus alcuronium is observed to have a much shorter duration of neuromuscular blocking action than its parent C-toxiferine I. [3] It also has a more rapid onset of action, and is ~1.5 times as potent as tubocurarine. [4] The pharmacological action of alcuronium is readily reversed by neostigmine, and it produces little histamine release. [5] The major disadvantage of alcuronium is that it elicits a vagolytic effect produced by a selective atropine-like blockade of cardiac muscarinic receptors. [4] [6] [7]