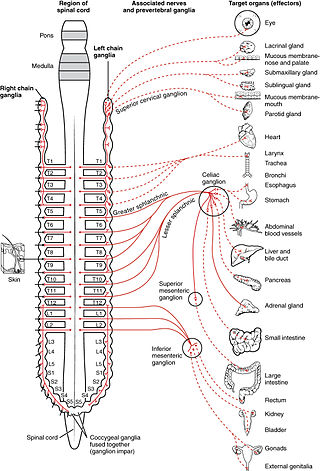
The sympathetic nervous system (SNS) is one of the three divisions of the autonomic nervous system, the others being the parasympathetic nervous system and the enteric nervous system. The enteric nervous system is sometimes considered part of the autonomic nervous system, and sometimes considered an independent system.
Anticholinergics are substances that block the action of the neurotransmitter called acetylcholine (ACh) at synapses in the central and peripheral nervous system.
Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system.

Tubocurarine is a toxic benzylisoquinoline alkaloid historically known for its use as an arrow poison. In the mid-1900s, it was used in conjunction with an anesthetic to provide skeletal muscle relaxation during surgery or mechanical ventilation. Safer alternatives, such as cisatracurium and rocuronium, have largely replaced it as an adjunct for clinical anesthesia and it is now rarely used.
Intrinsically photosensitive retinal ganglion cells (ipRGCs), also called photosensitive retinal ganglion cells (pRGC), or melanopsin-containing retinal ganglion cells (mRGCs), are a type of neuron in the retina of the mammalian eye. The presence of ipRGCs was first suspected in 1927 when rodless, coneless mice still responded to a light stimulus through pupil constriction, This implied that rods and cones are not the only light-sensitive neurons in the retina. Yet research on these cells did not advance until the 1980s. Recent research has shown that these retinal ganglion cells, unlike other retinal ganglion cells, are intrinsically photosensitive due to the presence of melanopsin, a light-sensitive protein. Therefore, they constitute a third class of photoreceptors, in addition to rod and cone cells.
Hexamethonium is a non-depolarising ganglionic blocker, a neuronal nicotinic (nAChR) receptor antagonist that acts in autonomic ganglia by binding mostly in or on the nAChR receptor, and not the acetylcholine binding site itself. It does not have any effect on the muscarinic acetylcholine receptors (mAChR) located on target organs of the parasympathetic nervous system, nor on the nicotinic receptors at the skeletal neuromuscular junction, but acts as antagonist at the nicotinic acetylcholine receptors located in sympathetic and parasympathetic ganglia (nAChR).

Arecoline is a nicotinic acid-based mild parasympathomimetic stimulant alkaloid found in the areca nut, the fruit of the areca palm. It is an odourless oily liquid. It can bring a sense of enhanced alertness and energy along with mild feelings of euphoria and relaxation. The psychoactive effects are comparable to that of nicotine.
Triethylcholine is a drug which mimics choline, and causes failure of cholinergic transmission by interfering with synthesis of acetylcholine in nerve endings.
A ganglionic blocker is a type of medication that inhibits transmission between preganglionic and postganglionic neurons in the autonomic nervous system, often by acting as a nicotinic receptor antagonist. Nicotinic acetylcholine receptors are found on skeletal muscle, but also within the route of transmission for the parasympathetic and sympathetic nervous system. More specifically, nicotinic receptors are found within the ganglia of the autonomic nervous system, allowing outgoing signals to be transmitted from the presynaptic to the postsynaptic cells. Thus, for example, blocking nicotinic acetylcholine receptors blocks both sympathetic (excitatory) and parasympathetic (calming) stimulation of the heart. The nicotinic antagonist hexamethonium, for example, does this by blocking the transmission of outgoing signals across the autonomic ganglia at the postsynaptic nicotinic acetylcholine receptor.

Prostaglandin F receptor (FP) is a receptor belonging to the prostaglandin (PG) group of receptors. FP binds to and mediates the biological actions of Prostaglandin F2α (PGF2α). It is encoded in humans by the PTGFR gene.
Pentolinium is a ganglionic blocking agent which acts as a nicotinic acetylcholine receptor antagonist. Formulated as the pentolinium tartrate salt, it is also known as Ansolysen. It can be used as an antihypertensive drug during surgery or to control hypertensive crises. It works by binding to the acetylcholine receptor of adrenergic nerves and thereby inhibiting the release of noradrenaline and adrenaline. Blocking this receptor leads to smooth muscle relaxation and vasodilation.

Surugatoxin (SGTX) is a type of venom found in the mid-gut digestive gland of the Japanese ivory mollusk Babyloniajaponica, a carnivorous gastropod. It functions as a ganglionic blocker of nicotinic acetylcholine receptors (nAChRs). The structurally and functionally related neosurugatoxin, also derived from Babylonia japonica, is an even more potent nAChR antagonist than SGTX.
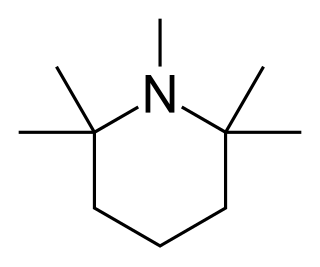
Pempidine is a ganglion-blocking drug, first reported in 1958 by two research groups working independently, and introduced as an oral treatment for hypertension.

Blonanserin, sold under the brand name Lonasen, is a relatively new atypical antipsychotic commercialized by Dainippon Sumitomo Pharma in Japan and Korea for the treatment of schizophrenia. Relative to many other antipsychotics, blonanserin has an improved tolerability profile, lacking side effects such as extrapyramidal symptoms, excessive sedation, or hypotension. As with many second-generation (atypical) antipsychotics it is significantly more efficacious in the treatment of the negative symptoms of schizophrenia compared to first-generation (typical) antipsychotics such as haloperidol.

Tertiapin is a 21-amino acid peptide isolated from venom of the European honey bee. It blocks two different types of potassium channels, inward rectifier potassium channels (Kir) and calcium activated large conductance potassium channels (BK).
The alpha-3 beta-4 nicotinic receptor, also known as the α3β4 receptor and the ganglion-type nicotinic receptor, is a type of nicotinic acetylcholine receptor, consisting of α3 and β4 subunits. It is located in the autonomic ganglia and adrenal medulla, where activation yields post- and/or presynaptic excitation, mainly by increased Na+ and K+ permeability.
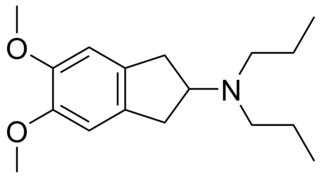
PNU-99,194(A) (or U-99,194(A)) is a drug which acts as a moderately selective D3 receptor antagonist with ~15-30-fold preference for D3 over the D2 subtype. Though it has substantially greater preference for D3 over D2, the latter receptor does still play some role in its effects, as evidenced by the fact that PNU-99,194 weakly stimulates both prolactin secretion and striatal dopamine synthesis, actions it does not share with the more selective (100-fold) D3 receptor antagonists S-14,297 and GR-103,691.

Candicine is a naturally occurring organic compound that is a quaternary ammonium salt with a phenethylamine skeleton. It is the N,N,N-trimethyl derivative of the well-known biogenic amine tyramine, and, being a natural product with a positively charged nitrogen atom in its molecular structure, it is classed as an alkaloid. Although it is found in a variety of plants, including barley, its properties have not been extensively studied with modern techniques. Candicine is toxic after parenteral administration, producing symptoms of neuromuscular blockade; further details are given in the "Pharmacology" section below.
A channel modulator, or ion channel modulator, is a type of drug which modulates ion channels. They include channel blockers and channel openers.

Cholinergic blocking drugs are a group of drugs that block the action of acetylcholine (ACh), a neurotransmitter, in synapses of the cholinergic nervous system. They block acetylcholine from binding to cholinergic receptors, namely the nicotinic and muscarinic receptors.














