
In biochemistry, a cholinesterase or choline esterase is a family of esterases that lyses choline-based esters, several of which serve as neurotransmitters. Thus, it is either of two enzymes that catalyze the hydrolysis of these cholinergic neurotransmitters, such as breaking acetylcholine into choline and acetic acid. These reactions are necessary to allow a cholinergic neuron to return to its resting state after activation. For example, in muscle contraction, acetylcholine at a neuromuscular junction triggers a contraction; but for the muscle to relax afterward, rather than remaining locked in a tense state, the acetylcholine must be broken down by a choline esterase. The main type for that purpose is acetylcholinesterase ; it is found mainly in chemical synapses and red blood cell membranes. The other type is butyrylcholinesterase ; it is found mainly in the blood plasma.
A parasympathomimetic drug, sometimes called a cholinomimetic drug or cholinergic receptor stimulating agent, is a substance that stimulates the parasympathetic nervous system (PSNS). These chemicals are also called cholinergic drugs because acetylcholine (ACh) is the neurotransmitter used by the PSNS. Chemicals in this family can act either directly by stimulating the nicotinic or muscarinic receptors, or indirectly by inhibiting cholinesterase, promoting acetylcholine release, or other mechanisms.

Galantamine is used for the treatment of cognitive decline in mild to moderate Alzheimer's disease and various other memory impairments. It is an alkaloid that has been isolated from the bulbs and flowers of Galanthus caucasicus, Galanthus woronowii, and some other members of the family Amaryllidaceae, such as Narcissus (daffodil), Leucojum aestivum (snowflake), and Lycoris including Lycoris radiata. It can also be produced synthetically.

SB-277,011A is a drug which acts as a potent and selective dopamine D3 receptor antagonist, which is around 80-100x selective for D3 over D2, and lacks any partial agonist activity.

Acetylcholinesterase, also known as AChE or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and of some other choline esters that function as neurotransmitters. AChE is found at mainly neuromuscular junctions and in chemical synapses of the cholinergic type, where its activity serves to terminate synaptic transmission. It belongs to carboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides.

The alpha-7 nicotinic receptor, also known as the α7 receptor, is a type of nicotinic acetylcholine receptor implicated in long term memory, consisting entirely of α7 subunits. As with other nicotinic acetylcholine receptors, functional α7 receptors are pentameric [i.e., (α7)5 stoichiometry].
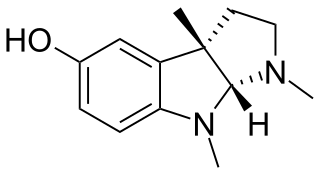
Eseroline is a drug which acts as an opioid agonist. It is a metabolite of the acetylcholinesterase inhibitor physostigmine but unlike physostigmine, the acetylcholinesterase inhibition produced by eseroline is weak and easily reversible, and it produces fairly potent analgesic effects mediated through the μ-opioid receptor. This mixture of activities gives eseroline an unusual pharmacological profile, although its uses are limited by side effects such as respiratory depression and neurotoxicity.

2-Methyl-6-(phenylethynyl)pyridine (MPEP) is a research drug which was one of the first compounds found to act as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. After being originally patented as a liquid crystal for LCDs, it was developed by the pharmaceutical company Novartis in the late 1990s. It was found to produce neuroprotective effects following acute brain injury in animal studies, although it was unclear whether these results were purely from mGluR5 blockade as it also acts as a weak NMDA antagonist, and as a positive allosteric modulator of another subtype mGlu4, and there is also evidence for a functional interaction between mGluR5 and NMDA receptors in the same populations of neurons. It was also shown to produce antidepressant and anxiolytic effects in animals, and to reduce the effects of morphine withdrawal, most likely due to direct interaction between mGluR5 and the μ-opioid receptor.

BW-723C86 is a tryptamine derivative drug which acts as a 5-HT2B receptor agonist. It has anxiolytic effects in animal studies, and is also used for investigating the function of the 5-HT2B receptor in a range of other tissues.

Acetylcholinesterase is the enzyme that is the primary member of the cholinesterase enzyme family. An acetylcholinesterase inhibitor (AChEI) is the inhibitor that inhibits acetylcholinesterase from breaking down acetylcholine into choline and acetate, thereby increasing both the level and duration of action of the neurotransmitter acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors.
Cholinesterase inhibitors, also known as anti-cholinesterase, are chemicals that prevent the breakdown of the neurotransmitter acetylcholine or butyrylcholine. This increases the amount of the acetylcholine or butyrylcholine in the synaptic cleft that can bind to muscarinic receptors, nicotinic receptors and others. This group of inhibitors are divided into two subgroups, acetylcholinesterase inhibitors and butyrylcholinesterase inhibitors.

Ladostigil (TV-3,326) is a novel neuroprotective agent being investigated for the treatment of neurodegenerative disorders like Alzheimer's disease, Lewy body disease, and Parkinson's disease. It acts as a reversible acetylcholinesterase and butyrylcholinesterase inhibitor, and an irreversible monoamine oxidase B inhibitor, and combines the mechanisms of action of older drugs like rivastigmine and rasagiline into a single molecule. In addition to its neuroprotective properties, ladostigil enhances the expression of neurotrophic factors like GDNF and BDNF, and may be capable of reversing some of the damage seen in neurodegenerative diseases via the induction of neurogenesis. Ladostigil also has antidepressant effects, and may be useful for treating comorbid depression and anxiety often seen in such diseases as well.
The alpha-3 beta-4 nicotinic receptor, also known as the α3β4 receptor and the ganglion-type nicotinic receptor, is a type of nicotinic acetylcholine receptor, consisting of α3 and β4 subunits. It is located in the autonomic ganglia and adrenal medulla, where activation yields post- and/or presynaptic excitation, mainly by increased Na+ and K+ permeability.

Dextrallorphan (DXA) is an opioid derivative chemical of the morphinan class that is used in scientific research. It acts as a σ1 receptor agonist and NMDA receptor antagonist. It has no significant affinity for the σ2, μ-opioid, or δ-opioid receptor, or for the serotonin or norepinephrine transporter. As an NMDA receptor antagonist, in vivo, it is approximately twice as potent as dextromethorphan, and five-fold less potent than dextrorphan.
Methanesulfonyl fluoride (MSF) has long been known to be a potent inhibitor of acetylcholinesterase AChE, the enzyme that regulates acetylcholine, an important neurotransmitter in both the central and peripheral nervous systems.

Ro 3-0422 is an extremely potent organophosphate acetylcholinesterase inhibitor. It is extremely toxic. The intravenous LD50 is 20 μg/kg in mice. It is over 300 times more potent than neostigmine.

Ro 3-0419 is a highly toxic organophosphate acetylcholinesterase inhibitor. It's the neutral analog of Ro 3-0422. Although Ro 3-0419 is less potent than Ro 3-0422, it does not have a positively charged nitrogen atom, so it's able to cross the blood brain barrier to inhibit cholinesterases in the brain. The intravenous LD50 of Ro 3-0419 is 1 mg/kg in mice.

TL-1238 is an extremely potent carbamate acetylcholinesterase inhibitor. It has been shown to be more potent than neostigmine.

GD-42 (methylsulfomethylate) is an irreversible acetylcholinesterase inhibitor. It has a positively charged sulfonium group.
Ro 3-0412 is an acetylcholinesterase inhibitor. It is the organophosphate analog of neostigmine.
















