
The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:

Physostigmine is a highly toxic parasympathomimetic alkaloid, specifically, a reversible cholinesterase inhibitor. It occurs naturally in the Calabar bean and the fruit of the Manchineel tree.
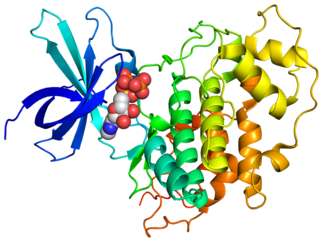
Glycogen synthase kinase 3 (GSK-3) is a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues. First discovered in 1980 as a regulatory kinase for its namesake, glycogen synthase (GS), GSK-3 has since been identified as a protein kinase for over 100 different proteins in a variety of different pathways. In mammals, including humans, GSK-3 exists in two isozymes encoded by two homologous genes GSK-3α (GSK3A) and GSK-3β (GSK3B). GSK-3 has been the subject of much research since it has been implicated in a number of diseases, including type 2 diabetes, Alzheimer's disease, inflammation, cancer, addiction and bipolar disorder.

Beta-secretase 1, also known as beta-site amyloid precursor protein cleaving enzyme 1, beta-site APP cleaving enzyme 1 (BACE1), membrane-associated aspartic protease 2, memapsin-2, aspartyl protease 2, and ASP2, is an enzyme that in humans is encoded by the BACE1 gene. Expression of BACE1 is observed mainly in neurons and oligodendrocytes.

Bulbocapnine is an alkaloid found in Corydalis and Dicentra, genera of the plant family Fumariaceae which have caused the fatal poisoning of sheep and cattle. It has been shown to act as an acetylcholinesterase inhibitor, and inhibits biosynthesis of dopamine via inhibition of the enzyme tyrosine hydroxylase. Like apomorphine, it is reported to be an inhibitor of amyloid beta protein (Aβ) fiber formation, whose presence is a hallmark of Alzheimer's disease (AD). Bulbocapnine is thus a potential therapeutic under the amyloid hypothesis. According to the Dorlands Medical Dictionary, it "inhibits the reflex and motor activities of striated muscle. It has been used in the treatment of muscular tremors and vestibular nystagmus".
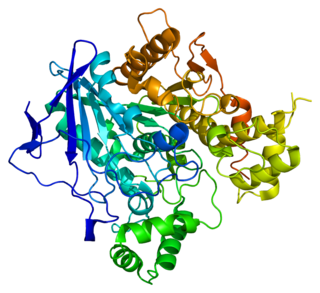
Butyrylcholinesterase, also known asBChE, BuChE, BuChase, pseudocholinesterase, or plasma (cholin)esterase, is a nonspecific cholinesterase enzyme that hydrolyses many different choline-based esters. In humans, it is made in the liver, found mainly in blood plasma, and encoded by the BCHE gene.

Monoamine oxidase B (MAO-B) is an enzyme that in humans is encoded by the MAOB gene.

Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters:

NEDD8-activating enzyme E1 regulatory subunit is a protein that in humans is encoded by the NAE1 gene.
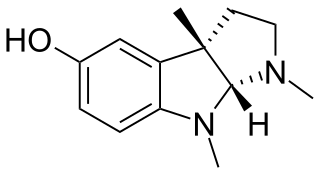
Eseroline is a drug which acts as an opioid agonist. It is a metabolite of the acetylcholinesterase inhibitor physostigmine but unlike physostigmine, the acetylcholinesterase inhibition produced by eseroline is weak and easily reversible, and it produces fairly potent analgesic effects mediated through the μ-opioid receptor. This mixture of activities gives eseroline an unusual pharmacological profile, although its uses are limited by side effects such as respiratory depression and neurotoxicity.

Baicalein (5,6,7-trihydroxyflavone) is a flavone, a type of flavonoid, originally isolated from the roots of Scutellaria baicalensis and Scutellaria lateriflora. It is also a constituent of Oroxylum indicum and thyme. It is the aglycone of baicalin.

Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family.

Cholinesterase inhibitors (ChEIs), also known as anti-cholinesterase, are chemicals that prevent the breakdown of the neurotransmitter acetylcholine or butyrylcholine by cholinesterase. This increases the amount of the acetylcholine or butyrylcholine in the synaptic cleft that can bind to muscarinic receptors, nicotinic receptors and others. This group of inhibitors is divided into two subgroups, acetylcholinesterase inhibitors (AChEIs) and butyrylcholinesterase inhibitors (BChEIs).

Ladostigil is a novel neuroprotective agent being investigated for the treatment of neurodegenerative disorders like Alzheimer's disease, Lewy body disease, and Parkinson's disease. It was developed from structural modification of rasagiline.

A phosphodiesterase-4 inhibitor, commonly referred to as a PDE4 inhibitor, is a drug used to block the degradative action of phosphodiesterase 4 (PDE4) on cyclic adenosine monophosphate (cAMP). It is a member of the larger family of PDE inhibitors. The PDE4 family of enzymes are the most prevalent PDE in immune cells. They are predominantly responsible for hydrolyzing cAMP within both immune cells and cells in the central nervous system.

Rivastigmine, sold under the brand name Exelon among others, is an acetylcholinesterase inhibitor used for the treatment of dementia associated with Alzheimer's disease and with Parkinson's disease. Rivastigmine can be administered orally or via a transdermal patch; the latter form reduces the prevalence of side effects, which typically include nausea and vomiting.
Methanesulfonyl fluoride (MSF) has long been known to be a potent inhibitor of acetylcholinesterase (AChE), the enzyme that regulates acetylcholine, an important neurotransmitter in both the central and peripheral nervous systems.
Phosphodiesterases (PDEs) are a superfamily of enzymes. This superfamily is further classified into 11 families, PDE1 - PDE11, on the basis of regulatory properties, amino acid sequences, substrate specificities, pharmacological properties and tissue distribution. Their function is to degrade intracellular second messengers such as cyclic adenine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) which leads to several biological processes like effect on intracellular calcium level by the Ca2+ pathway.

Phenserine is a synthetic drug which has been investigated as a medication to treat Alzheimer's disease (AD), as the drug exhibits neuroprotective and neurotrophic effects.

Buntanetap is an orally-administered small molecule inhibitor of several neurotoxic proteins that is under investigation in the treatment of Alzheimer's disease, frontotemporal dementia, chronic traumatic encephalopathy and Parkinson's disease. It is the (+) enantiomer of phenserine, as the (-) enantiomer also has unwanted anticholinergic effects. It is currently in phase III trials for the treatment of Parkinson's.



















