
Voacangine is an alkaloid found predominantly in the root bark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides, Tabernaemontana divaricata and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Tabernaemontana corymbosa is a species of plant in the family Apocynaceae. It is found in Brunei, China, Indonesia, Laos, Malaysia, Myanmar, Singapore, Thailand, and Vietnam. Glossy green leaves and faintly sweet scented flower. Flowers continuously all year. Frost tolerant. Grows to about 2metres. Likes full sun to part shade. A number of cultivars are available.
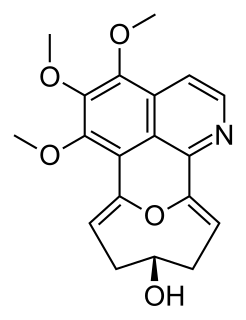
Eletefine is an isoquinoline alkaloid first isolated in 1998 from Cissampelos glaberrima. It is one of few known compounds containing the so-called stephaoxocane skeleton, alongside stephaoxocanidine, excentricine, and the stephalonganines.

Voacamine, also known under the older names voacanginine and vocamine, is a naturally occurring dimeric indole alkaloid of the secologanin type, found in a number of plants, including Voacanga africana and Tabernaemontana divaricata. It is approved for use as an antimalarial drug in several African countries. Voacamine exhibits cannabinoid CB1 receptor antagonistic activity.

Tabernaemontana elegans, the toad tree, is a shrub or small tree in the family Apocynaceae. It is native to eastern Africa.

Conodurine is an acetylcholinesterase inhibitor and butyrylcholinesterase inhibitor isolated from Tabernaemontana.
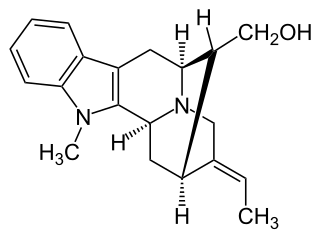
Affinisine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana. Structurally, it can be considered a member of the sarpagine alkaloid family and may be synthesized from tryptophan via a Pictet-Spengler reaction.

Stemmadenine is a terpene indole alkaloid. Stemmadenine is believed to be formed from preakuammicine by a carbon-carbon bond cleavage. Cleavage of a second carbon-carbon bond is thought to form dehydrosecodine. The enzymes forming stemmadenine and using it as a substrate remain unknown to date. It is thought to be intermediate compound in many different biosynthetic pathways such as in Aspidosperma species. Many alkaloids are proposed to be produced through intermediate stemmadenine. Some of them are:

Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.

Myrcia guianensis (pedra-ume-caá) is a species of the flowering plant family Myrtaceae. It is found in South America.

Annona dioica is a species of plant in the family Annonaceae. It is native to Bolivia, Brazil and Paraguay. Augustin Saint-Hilaire, the French botanist who first formally described the species, named it after its flowers which have different reproductive structures and.

Tabernaemontanine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Dregamine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Ervatamia hirta and Tabernaemontana divaricata.
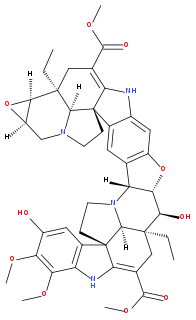
Conophylline is a autophagy inducing vinca alkaloid found in several species of Tabernaemontana including Ervatamia microphylla and Tabernaemontana divaricata. Among its many functional groups is an epoxide: the compound where that ring is replaced with a double bond is called conophyllidine and this co-occurs in the same plants.

Vobasine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Voacristine is a indole alkaloid occurring in Voacanga and Tabernaemontana genus. It is also an iboga type alkaloid.
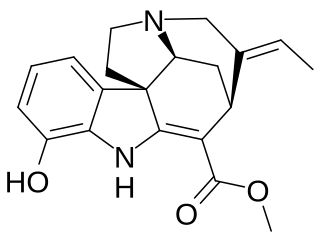
Vinervine is a monoterpene indole alkaloid of the Vinca sub-group. It is a derivative of akuammicine, with one additional hydroxy (OH) group in the indole portion, hence it is also known as 12-hydroxyakuammicine.
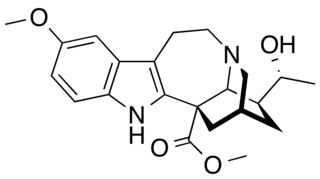
19-Epivoacristine is an indole alkaloid found in different species of Tabernaemontana, such as Tabernaemontana dichotoma, as well as in Peschiera affinis. It is also known as 20-epivoacangarine and 19-epi-voacangarine.

Conopharyngine is the major alkaloid present in the leaves and stem-bark of Tabernaemontana pachysiphon and Conopharyngia durissima. It is closely related voacangine and coronaridine. Conopharyngine pseudoindoxyl, a derivative of it, is also found in the same plant Tabernaemontana pachysiphon.




















