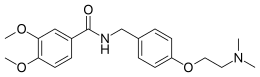
Metoclopramide is a medication used for stomach and esophageal problems. It is commonly used to treat and prevent nausea and vomiting, to help with emptying of the stomach in people with delayed stomach emptying, and to help with gastroesophageal reflux disease. It is also used to treat migraine headaches.

Donepezil, sold under the brand name Aricept among others, is a medication used to treat dementia of the Alzheimer's type. It appears to result in a small benefit in mental function and ability to function. Use, however, has not been shown to change the progression of the disease. Treatment should be stopped if no benefit is seen. It is taken by mouth or via a transdermal patch.

Cisapride is a gastroprokinetic agent, a drug that increases motility in the upper gastrointestinal tract. It acts directly as a serotonin 5-HT4 receptor agonist and indirectly as a parasympathomimetic. Stimulation of the serotonin receptors increases acetylcholine release in the enteric nervous system. It has been sold under the trade names Prepulsid (Janssen-Ortho) and Propulsid (in the United States). It was discovered by Janssen Pharmaceuticals in 1980. In many countries, it has been either withdrawn from the market or had its indications limited due to incidence of serious cardiac side-effects. Propulsid was linked to children's deaths.
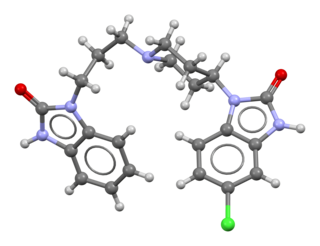
Domperidone, sold under the brand name Motilium among others, is a dopamine antagonist medication which is used to treat nausea and vomiting and certain gastrointestinal problems like gastroparesis. It raises the level of prolactin in the human body and is used off label to induce and promote breast milk production. It may be taken by mouth or rectally.

Ondansetron, sold under the brand name Zofran among others, is a medication used to prevent nausea and vomiting caused by chemotherapy, radiation therapy, migraines, or surgery. It is also effective for treating gastroenteritis. It can be given orally, intramuscularly, or intravenously.
Indigestion, also known as dyspepsia or upset stomach, is a condition of impaired digestion. Symptoms may include upper abdominal fullness, heartburn, nausea, belching, or upper abdominal pain. People may also experience feeling full earlier than expected when eating. Indigestion is relatively common, affecting 20% of people at some point during their life, and is frequently caused by gastroesophageal reflux disease (GERD) or gastritis.

Functional dyspepsia (FD) is a common gastrointestinal disorder defined by symptoms arising from the gastroduodenal region in the absence of an underlying organic disease that could easily explain the symptoms. Characteristic symptoms include epigastric burning, epigastric pain, postprandial fullness, and early satiety. FD was formerly known as non-ulcer dyspepsia, as opposed to "organic dyspepsia" with underlying conditions of gastritis, peptic ulcer disease, or cancer.
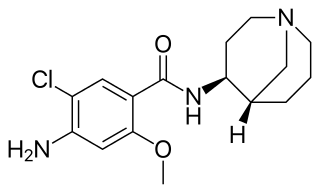
Renzapride is a prokinetic agent and antiemetic which acts as a full 5-HT4 agonist and partial 5-HT3 antagonist. It also functions as a 5-HT2B antagonist and has some affinity for the 5-HT2A and 5-HT2C receptors.
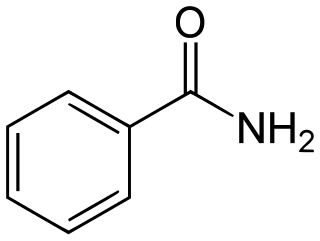
Benzamide is an organic compound with the chemical formula of C7H7NO. It is the simplest amide derivative of benzoic acid. In powdered form, it appears as a white solid, while in crystalline form, it appears as colourless crystals. It is slightly soluble in water, and soluble in many organic solvents. It is a natural alkaloid found in the herbs of Berberis pruinosa.

Gastroparesis is a medical disorder of ineffective neuromuscular contractions (peristalsis) of the stomach, resulting in food and liquid remaining in the stomach for a prolonged period of time. Stomach contents thus exit more slowly into the duodenum of the digestive tract, a medical sign called delayed gastric emptying. The opposite of this, where stomach contents exit quickly into the duodenum, is called dumping syndrome.
Mosapride is a gastroprokinetic agent that acts as a selective 5HT4 agonist. The major active metabolite of mosapride, known as M1, additionally acts as a 5HT3 antagonist, which accelerates emptying throughout the whole of the gastrointestinal tract in humans, and is used for the treatment of gastritis, gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome. It is recommended to be taken on an empty stomach (i.e. at least one hour before food or two hours after food).
Iberogast, also known as STW5, is a liquid formulation of nine herbs used for functional dyspepsia and irritable bowel syndrome. A proprietary blend, it was developed in Germany in 1961 and is available in other countries. Named after the genus (Iberis) of one of its ingredients, it is also claimed to possess anti-inflammatory, antioxidative and free radical–inhibiting properties as well as the ability to reduce gastric acid secretion.
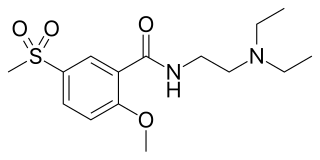
Tiapride is a drug that selectively blocks D2 and D3 dopamine receptors in the brain. It is used to treat a variety of neurological and psychiatric disorders including dyskinesia, alcohol withdrawal syndrome, negative symptoms of psychosis, and agitation and aggression in the elderly. A derivative of benzamide, tiapride is chemically and functionally similar to other benzamide antipsychotics such as sulpiride and amisulpride known for their dopamine antagonist effects.

Dexloxiglumide is a drug which acts as a cholecystokinin antagonist, selective for the CCKA subtype. It inhibits gastrointestinal motility and reduces gastric secretions, and despite older selective CCKA antagonists such as lorglumide and devazepide having had only limited success in trials and ultimately never making it into clinical use, dexloxiglumide is being investigated as a potential treatment for a variety of gastrointestinal problems including irritable bowel syndrome, dyspepsia, constipation and pancreatitis, and has had moderate success so far although trials are still ongoing.

Prucalopride, sold under brand names Resolor and Motegrity among others, is a medication acting as a selective, high affinity 5-HT4 receptor agonist which targets the impaired motility associated with chronic constipation, thus normalizing bowel movements. Prucalopride was approved for medical use in the European Union in 2009, in Canada in 2011, in Israel in 2014, and in the United States in December 2018. The drug has also been tested for the treatment of chronic intestinal pseudo-obstruction.

Cinitapride (trade names Cintapro, Pemix) is a gastroprokinetic agent and antiemetic agent of the benzamide class which is marketed in India, Mexico, Pakistan and Spain. It acts as an agonist of the 5-HT1 and 5-HT4 receptors and as an antagonist of the 5-HT2 receptors.
A prokinetic agent is a type of drug which enhances gastrointestinal motility by increasing the frequency or strength of contractions, but without disrupting their rhythm. They are used to treat certain gastrointestinal symptoms, including abdominal discomfort, bloating, constipation, heart burn, nausea, and vomiting; and certain gastrointestinal disorders, including irritable bowel syndrome, gastritis, gastroparesis, and functional dyspepsia.
Cariprazine, sold under the brand name Vraylar among others, is an atypical antipsychotic developed by Gedeon Richter, which is used in the treatment of schizophrenia, bipolar mania, bipolar depression, and major depressive disorder. It acts primarily as a D3 and D2 receptor partial agonist, with a preference for the D3 receptor. Cariprazine is also a partial agonist at the serotonin 5-HT1A receptor and acts as an antagonist at 5-HT2B and 5-HT2A receptors. It is taken by mouth. The most prevalent side effects include nausea, mild sedation, fatigue, and dizziness. At higher dosages, there is an increased risk for restlessness, insomnia, and tremors.

GR-113808 is a drug which acts as a potent and selective 5-HT4 serotonin receptor antagonist. It is used in researching the roles of 5-HT4 receptors in various processes, and has been used to test some of the proposed therapeutic effects of selective 5-HT4 agonists, such as for instance blocking the nootropic effects of 5-HT4 agonists, and worsening the respiratory depression produced by opioid analgesic drugs, which appears to be partly 5-HT4 mediated and can be counteracted by certain 5-HT4 agonists.
CJ-033466 is a drug which acts as a potent and selective 5-HT4 serotonin receptor partial agonist. In animal tests it stimulated gastrointestinal motility with 30 times the potency of cisapride, and with lower affinity for the hERG channel.