
Azapirones are a class of drugs used as anxiolytics, antidepressants, and antipsychotics. They are commonly used as add-ons to other antidepressants, such as selective serotonin reuptake inhibitors (SSRIs).

A dopamine antagonist, also known as an anti-dopaminergic and a dopamine receptor antagonist (DRA), is a type of drug which blocks dopamine receptors by receptor antagonism. Most antipsychotics are dopamine antagonists, and as such they have found use in treating schizophrenia, bipolar disorder, and stimulant psychosis. Several other dopamine antagonists are antiemetics used in the treatment of nausea and vomiting.

Amisulpride, sold under the brand names Solian and Barhemsys, is a medication used in the treatment of schizophrenia, acute psychotic episodes, depression, and nausea and vomiting. It is specifically used at lower doses intravenously to prevent and treat postoperative nausea and vomiting; at low doses by mouth to treat depression; and at higher doses by mouth to treat psychosis.

Molindone, sold under the brand name Moban, is an antipsychotic medication which is used in the United States in the treatment of schizophrenia. It is taken by mouth.

Iloperidone, commonly known as Fanapt and previously known as Zomaril, is an atypical antipsychotic for the treatment of schizophrenia.
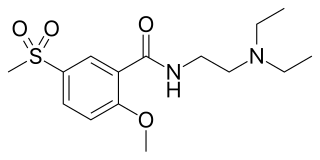
Tiapride is a drug that selectively blocks D2 and D3 dopamine receptors in the brain. It is used to treat a variety of neurological and psychiatric disorders including dyskinesia, alcohol withdrawal syndrome, negative symptoms of psychosis, and agitation and aggression in the elderly. A derivative of benzamide, tiapride is chemically and functionally similar to other benzamide antipsychotics such as sulpiride and amisulpride known for their dopamine antagonist effects.

Sultopride (trade names Barnetil, Barnotil, Topral) is an atypical antipsychotic of the benzamide chemical class used in Europe, Japan, and Hong Kong for the treatment of schizophrenia. It was launched by Sanofi-Aventis in 1976. Sultopride acts as a selective D2 and D3 receptor antagonist. It has also been shown to have clinically relevant affinity for the GHB receptor as well, a property it shares in common with amisulpride and sulpiride.

Perospirone (Lullan) is an atypical antipsychotic of the azapirone family. It was introduced in Japan by Dainippon Sumitomo Pharma in 2001 for the treatment of schizophrenia and acute cases of bipolar mania.

Blonanserin, sold under the brand name Lonasen, is a relatively new atypical antipsychotic commercialized by Dainippon Sumitomo Pharma in Japan and Korea for the treatment of schizophrenia. Relative to many other antipsychotics, blonanserin has an improved tolerability profile, lacking side effects such as extrapyramidal symptoms, excessive sedation, or hypotension. As with many second-generation (atypical) antipsychotics it is significantly more efficacious in the treatment of the negative symptoms of schizophrenia compared to first-generation (typical) antipsychotics such as haloperidol.

Mosapramine (Cremin) is an atypical antipsychotic used in Japan for the treatment of schizophrenia. It is a potent dopamine antagonist with high affinity to the D2, D3, and D4 receptors, and with moderate affinity for the 5-HT2 receptors.
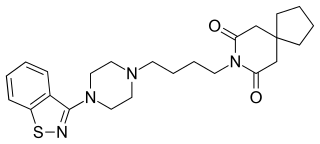
Tiospirone (BMY-13,859), also sometimes called tiaspirone or tiosperone, is an atypical antipsychotic of the azapirone class. It was investigated as a treatment for schizophrenia in the late 1980s and was found to have an effectiveness equivalent to those of typical antipsychotics in clinical trials but without causing extrapyramidal side effects. However, development was halted and it was not marketed. Perospirone, another azapirone derivative with antipsychotic properties, was synthesized and assayed several years after tiospirone. It was found to be both more potent and more selective in comparison and was commercialized instead.
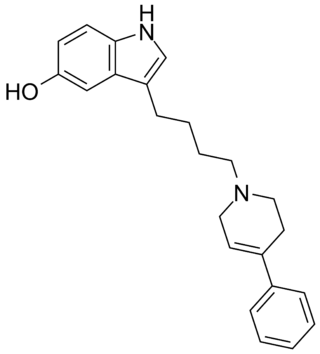
Roxindole (EMD-49,980) is a dopaminergic and serotonergic drug which was originally developed by Merck KGaA for the treatment of schizophrenia. In clinical trials its antipsychotic efficacy was only modest but it was unexpectedly found to produce potent and rapid antidepressant and anxiolytic effects. As a result, roxindole was further researched for the treatment of depression instead. It has also been investigated as a therapy for Parkinson's disease and prolactinoma.

Clocapramine, also known as 3-chlorocarpipramine, is an atypical antipsychotic of the iminostilbene class which was introduced in Japan in 1974 by Yoshitomi for the treatment of schizophrenia. In addition to psychosis, clocapramine has also been used to augment antidepressants in the treatment of anxiety and panic.

Mazapertine (RWJ-37796) is an antipsychotic agent that was developed by Johnson & Johnson but never marketed. It exerts its pharmacological effect through affinity for dopamine D2, serotonin 5-HT1A, and α1-adrenergic receptors.

F-15,063 is an orally active potential antipsychotic, and an antagonist at the D2/D3 receptors, partial agonist at the D4 receptor, and agonist at the 5-HT1A receptors. It has greater efficacy at the 5-HT1A receptors than other antipsychotics, such as clozapine, aripiprazole, and ziprasidone. This greater efficacy may lead to enhanced antipsychotic properties, as antipsychotics that lack 5-HT1A affinity are associated with increased risk of extrapyramidal symptoms, and lack of activity against the negative symptoms of schizophrenia.

Brexpiprazole, sold under the brand name Rexulti among others, is an atypical antipsychotic medication used for the treatment of major depressive disorder, schizophrenia, and agitation associated with dementia due to Alzheimer's disease.

Brilaroxazine, also known as oxaripiprazole, is an investigational atypical antipsychotic which is under development by Reviva Pharmaceuticals for the treatment of neuropsychiatric and inflammatory disorders. It has currently completed the first of two phase III clinical trials for schizophrenia. Reviva Pharmaceuticals also intends to investigate brilaroxazine for the treatment of bipolar disorder, major depressive disorder, attention deficit hyperactivity disorder (ADHD), irritability in autism, tics, psychosis/agitation associated with Alzheimer's disease, Parkinson's disease psychosis, as well as the inflammatory disorders pulmonary arterial hypertension (PAH), idiopathic pulmonary fibrosis (IPF), and psoriasis. The FDA granted brilaroxazine orphan drug designation for the treatment of PAH and IPF.

Aripiprazole lauroxil, sold under the brand name Aristada, is a long-acting injectable atypical antipsychotic that was developed by Alkermes. It is an N-acyloxymethyl prodrug of aripiprazole that is administered via intramuscular injection once every four to eight weeks for the treatment of schizophrenia. Aripiprazole lauroxil was approved by the U.S. Food and Drug Administration (FDA) on 5 October 2015.
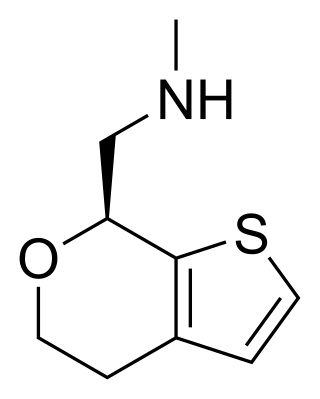
Ulotaront is an investigational antipsychotic that is undergoing clinical trials for the treatment of schizophrenia and Parkinson's disease psychosis. The medication was discovered in collaboration between PsychoGenics Inc. and Sunovion Pharmaceuticals using PsychoGenics' behavior and AI-based phenotypic drug discovery platform, SmartCube. Ulotaront is in Phase III of clinical development.

ENX-104, also known as deuterated nemonapride enantiomer, is a selective dopamine D2 and D3 receptor antagonist which is under development for the treatment of major depressive disorder. It is specifically under development for the treatment of major depressive disorder characterized by anhedonia. The drug is being developed for use at low doses to preferentially block presynaptic dopamine D2 and D3 autoreceptors and hence to enhance rather than inhibit dopaminergic neurotransmission. It is taken by mouth.




















