Haloperidol, sold under the brand name Haldol among others, is a typical antipsychotic medication. Haloperidol is used in the treatment of schizophrenia, tics in Tourette syndrome, mania in bipolar disorder, delirium, agitation, acute psychosis, and hallucinations from alcohol withdrawal. It may be used by mouth or injection into a muscle or a vein. Haloperidol typically works within 30 to 60 minutes. A long-acting formulation may be used as an injection every four weeks by people with schizophrenia or related illnesses, who either forget or refuse to take the medication by mouth.

The atypical antipsychotics (AAP), also known as second generation antipsychotics (SGAs) and serotonin–dopamine antagonists (SDAs), are a group of antipsychotic drugs largely introduced after the 1970s and used to treat psychiatric conditions. Some atypical antipsychotics have received regulatory approval for schizophrenia, bipolar disorder, irritability in autism, and as an adjunct in major depressive disorder.

Risperidone, sold under the brand name Risperdal among others, is an atypical antipsychotic used to treat schizophrenia and bipolar disorder, as well as irritability associated with autism. It is taken either by mouth or by injection. The injectable versions are long-acting and last for 2–4 weeks.

Quetiapine, sold under the brand name Seroquel among others, is an atypical antipsychotic medication used for the treatment of schizophrenia, bipolar disorder, and major depressive disorder. Despite being widely used as a sleep aid due to its sedating effect, the benefits of such use may not outweigh its undesirable side effects. It is taken orally.

Ziprasidone, sold under the brand name Geodon among others, is an atypical antipsychotic used to treat schizophrenia and bipolar disorder. It may be used by mouth and by injection into a muscle (IM). The IM form may be used for acute agitation in people with schizophrenia.
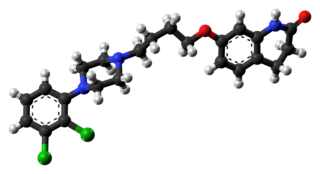
Aripiprazole, sold under the brand names Abilify and Aristada, among others, is an atypical antipsychotic. It is primarily used in the treatment of schizophrenia and bipolar disorder; other uses include as an add-on treatment in major depressive disorder and obsessive–compulsive disorder (OCD), tic disorders, and irritability associated with autism. Aripiprazole is taken by mouth or via injection into a muscle. A Cochrane review found low-quality evidence of effectiveness in treating schizophrenia.

Sertindole, sold under the brand name Serdolect among others, is an antipsychotic medication. Sertindole was developed by the Danish pharmaceutical company Lundbeck and marketed under license by Abbott Labs. Like other atypical antipsychotics, it has activity at dopamine and serotonin receptors in the brain. It is used in the treatment of schizophrenia. It is classified chemically as a phenylindole derivative.

Ketanserin (INN, USAN, BAN) (brand name Sufrexal; former developmental code name R41468) is a drug used clinically as an antihypertensive agent and in scientific research to study the serotonergic system; specifically, the 5-HT2 receptor family. It was discovered at Janssen Pharmaceutica in 1980. It is not available in the United States.

Tiotixene, or thiothixene is a typical antipsychotic agent currently sold under the brand name Navane which is predominantly utilised to treat acute and chronic schizophrenia. Beyond its primary indication, it can exhibit a variety of effects common to neuroleptic drugs including anxiolytic, anti-depressive, and anti-aggressive properties.

Amisulpride is an antiemetic and antipsychotic medication used at lower doses intravenously to prevent and treat postoperative nausea and vomiting; and at higher doses by mouth to treat schizophrenia and acute psychotic episodes. It is sold under the brand names Barhemsys and Solian, Socian, Deniban and others. At very low doses it is also used to treat dysthymia.
Butyrophenone is an organic compound with the formula C6H5C(O)C3H7. It is a colorless liquid.

Trifluperidol is a typical antipsychotic of the butyrophenone chemical class. It has general properties similar to those of haloperidol, but is considerably more potent by weight, and causes relatively more severe side effects, especially tardive dyskinesia and other extrapyramidal effects. It is used in the treatment of psychoses including mania and schizophrenia. It was discovered at Janssen Pharmaceutica in 1959.

Melperone is an atypical antipsychotic of the butyrophenone chemical class, making it structurally related to the typical antipsychotic haloperidol. It first entered clinical use in 1960s.

Flunoxaprofen, also known as Priaxim, is a chiral nonsteroidal anti-inflammatory drug (NSAID). It is closely related to naproxen, which is also an NSAID. Flunoxaprofen has been shown to significantly improve the symptoms of osteoarthritis and rheumatoid arthritis. The clinical use of flunoxaprofen has ceased due to concerns of potential hepatotoxicity.

Setoperone is a compound that is a ligand to the 5-HT2A receptor. It can be radiolabeled with the radioisotope fluorine-18 and used as a radioligand with positron emission tomography (PET). Several research studies have used the radiolabeled setoperone in neuroimaging for the studying neuropsychiatric disorders, such as depression or schizophrenia.
Lurasidone, sold under the brand name Latuda among others, is an antipsychotic medication used to treat schizophrenia and bipolar disorder. It is taken by mouth.

Volinanserin (INN) is a highly selective 5-HT2A receptor antagonist that is frequently used in scientific research to investigate the function of the 5-HT2A receptor. It was also tested in clinical trials as a potential antipsychotic, antidepressant, and treatment for insomnia but was never marketed.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.
Tiospirone (BMY-13,859), also sometimes called tiaspirone or tiosperone, is an atypical antipsychotic of the azapirone class. It was investigated as a treatment for schizophrenia in the late 1980s and was found to have an effectiveness equivalent to those of typical antipsychotics in clinical trials but without causing extrapyramidal side effects. However, development was halted and it was not marketed. Perospirone, another azapirone derivative with antipsychotic properties, was synthesized and assayed several years after tiospirone. It was found to be both more potent and more selective in comparison and was commercialized instead.

Mazapertine (RWJ-37796) is an antipsychotic agent that was developed by Johnson & Johnson but never marketed. It exerts its pharmacological effect through affinity for dopamine D2, serotonin 5-HT1A, and α1-adrenergic receptors.