
An acetylcholine receptor is an integral membrane protein that responds to the binding of acetylcholine, a neurotransmitter.

Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly C. dealbata. Mushrooms in the genera Entoloma and Mycena have also been found to contain levels of muscarine which can be dangerous if ingested. Muscarine has been found in harmless trace amounts in Boletus, Hygrocybe, Lactarius and Russula. Trace concentrations of muscarine are also found in Amanita muscaria, though the pharmacologically more relevant compound from this mushroom is the Z-drug-like alkaloid muscimol. A. muscaria fruitbodies contain a variable dose of muscarine, usually around 0.0003% fresh weight. This is very low and toxicity symptoms occur very rarely. Inocybe and Clitocybe contain muscarine concentrations up to 1.6%.

Chlorphenamine, also known as chlorpheniramine, is an antihistamine used to treat the symptoms of allergic conditions such as allergic rhinitis. It is taken by mouth. The medication takes effect within two hours and lasts for about 4-6 hours.
A parasympathomimetic drug, sometimes called a cholinomimetic drug or cholinergic receptor stimulating agent, is a substance that stimulates the parasympathetic nervous system (PSNS). These chemicals are also called cholinergic drugs because acetylcholine (ACh) is the neurotransmitter used by the PSNS. Chemicals in this family can act either directly by stimulating the nicotinic or muscarinic receptors, or indirectly by inhibiting cholinesterase, promoting acetylcholine release, or other mechanisms. Common uses of parasympathomimetics include glaucoma, Sjögren syndrome and underactive bladder.

Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system.

Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system.

Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine is the first observed example of a chlorinated alkaloid remains controversial, due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.

Neuromuscular-blocking drugs block neuromuscular transmission at the neuromuscular junction, causing paralysis of the affected skeletal muscles. This is accomplished via their action on the post-synaptic acetylcholine (Nm) receptors.
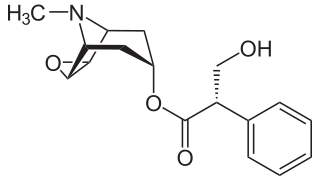
A muscarinic receptor antagonist (MRA) is a type of anticholinergic agent that blocks the activity of the muscarinic acetylcholine receptor. The muscarinic receptor is a protein involved in the transmission of signals through certain parts of the nervous system, and muscarinic receptor antagonists work to prevent this transmission from occurring. Notably, muscarinic antagonists reduce the activation of the parasympathetic nervous system. The normal function of the parasympathetic system is often summarised as "rest-and-digest", and includes slowing of the heart, an increased rate of digestion, narrowing of the airways, promotion of urination, and sexual arousal. Muscarinic antagonists counter this parasympathetic "rest-and-digest" response, and also work elsewhere in both the central and peripheral nervous systems.
A nicotinic agonist is a drug that mimics the action of acetylcholine (ACh) at nicotinic acetylcholine receptors (nAChRs). The nAChR is named for its affinity for nicotine.

The human muscarinic acetylcholine receptor M5, encoded by the CHRM5 gene, is a member of the G protein-coupled receptor superfamily of integral membrane proteins. It is coupled to Gq protein. Binding of the endogenous ligand acetylcholine to the M5 receptor triggers a number of cellular responses such as adenylate cyclase inhibition, phosphoinositide degradation, and potassium channel modulation. Muscarinic receptors mediate many of the effects of acetylcholine in the central and peripheral nervous system. The clinical implications of this receptor have not been fully explored; however, stimulation of this receptor is known to effectively decrease cyclic AMP levels and downregulate the activity of protein kinase A (PKA).

The muscarinic acetylcholine receptor M1, also known as the cholinergic receptor, muscarinic 1, is a muscarinic receptor that in humans is encoded by the CHRM1 gene. It is localized to 11q13.

The muscarinic acetylcholine receptor M2, also known as the cholinergic receptor, muscarinic 2, is a muscarinic acetylcholine receptor that in humans is encoded by the CHRM2 gene. Multiple alternatively spliced transcript variants have been described for this gene.

The muscarinic acetylcholine receptor, also known as cholinergic/acetylcholine receptor M3, or the muscarinic 3, is a muscarinic acetylcholine receptor encoded by the human gene CHRM3.

The muscarinic acetylcholine receptor M4, also known as the cholinergic receptor, muscarinic 4 (CHRM4), is a protein that, in humans, is encoded by the CHRM4 gene.

Xanomeline is a small molecule muscarinic acetylcholine receptor agonist that was first synthesized in a collaboration between Eli Lilly and Novo Nordisk as an investigational therapeutic being studied for the treatment of central nervous system disorders.
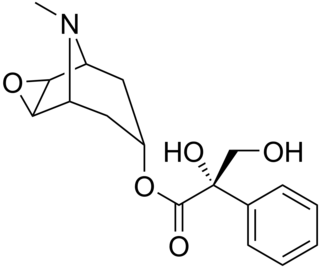
Anisodine, also known as daturamine and α-hydroxyscopolamine, is an antispasmodic and anticholinergic drug used in the treatment of acute circulatory shock in China. It is a tropane alkaloid and is found naturally in plants of the family Solanaceae - notably Anisodus tanguticus (syn. Scopolia tangutica. Anisodine acts as a muscarinic acetylcholine receptor antagonist and α1-adrenergic receptor antagonist.

PD-102,807 is a drug which acts as a selective antagonist for the muscarinic acetylcholine receptor M4. It is used in scientific research for studying the effects of the different muscarinic receptor subtypes in the body and brain.

Methoctramine is a polymethylene tetraamine that acts as a muscarinic antagonist. It preferentially binds to the pre-synaptic receptor M2, a muscarinic acetylcholine ganglionic protein complex present basically in heart cells. In normal conditions -absence of methoctramine-, the activation of M2 receptors diminishes the speed of conduction of the sinoatrial and atrioventricular nodes thus reducing the heart rate. Thanks to its apparently high cardioselectivity, it has been studied as a potential parasymphatolitic drug, particularly against bradycardia. However, currently it is only addressed for research purposes, since the administration to humans is still unavailable.
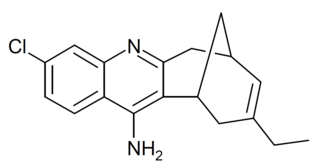
Huprine X is a synthetic cholinergic compound developed as a hybrid between the natural product Huperzine A and the synthetic drug tacrine. It is one of the most potent reversible inhibitors of acetylcholinesterase known, with a binding affinity of 0.026nM, as well as showing direct agonist activity at both nicotinic and muscarinic acetylcholine receptors. In animal studies it has nootropic and neuroprotective effects, and is used in research into Alzheimer's disease, and although huprine X itself has not been researched for medical use in humans, a large family of related derivatives have been developed.

















