This article needs additional citations for verification .(August 2016) |

In organic chemistry, a functional group is any substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. [1] [2] This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.
Contents
- Table of common functional groups
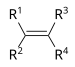
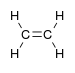
- Hydrocarbons
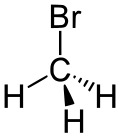
- Groups containing halogens
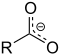
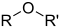
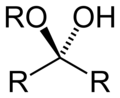
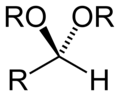
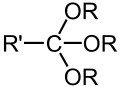
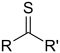
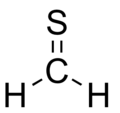
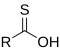
- Groups containing oxygen
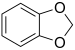
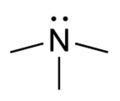
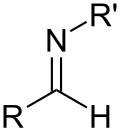
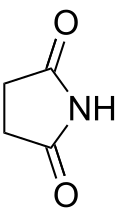
- Groups containing nitrogen
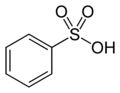
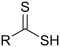
- Groups containing sulfur
- Groups containing phosphorus
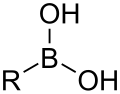
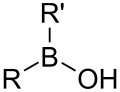
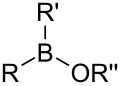
- Groups containing boron
- Groups containing metals
- Names of radicals or moieties
- See also
- References
- External links
A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Functional groups can also be charged, e.g. in carboxylate salts (−COO−), which turns the molecule into a polyatomic ion or a complex ion. Functional groups binding to a central atom in a coordination complex are called ligands . Complexation and solvation are also caused by specific interactions of functional groups. In the common rule of thumb "like dissolves like", it is the shared or mutually well-interacting functional groups which give rise to solubility. For example, sugar dissolves in water because both share the hydroxyl functional group (−OH) and hydroxyls interact strongly with each other. Plus, when functional groups are more electronegative than atoms they attach to, the functional groups will become polar, and the otherwise nonpolar molecules containing these functional groups become polar and so become soluble in some aqueous environment.
Combining the names of functional groups with the names of the parent alkanes generates what is termed a systematic nomenclature for naming organic compounds. In traditional nomenclature, the first carbon atom after the carbon that attaches to the functional group is called the alpha carbon; the second, beta carbon, the third, gamma carbon, etc. If there is another functional group at a carbon, it may be named with the Greek letter, e.g., the gamma-amine in gamma-aminobutyric acid is on the third carbon of the carbon chain attached to the carboxylic acid group. IUPAC conventions call for numeric labeling of the position, e.g. 4-aminobutanoic acid. In traditional names various qualifiers are used to label isomers, for example, isopropanol (IUPAC name: propan-2-ol) is an isomer of n-propanol (propan-1-ol). The term moiety has some overlap with the term "functional group". However, a moiety is an entire "half" of a molecule, which can be not only a single functional group, but also a larger unit consisting of multiple functional groups. For example, an "aryl moiety" may be any group containing an aromatic ring, regardless of how many functional groups the said aryl has.