
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.

Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure or mixing with a catalyst. Heat is not necessarily applied externally, and is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.

Phenol formaldehyde resins (PF) are synthetic polymers obtained by the reaction of phenol or substituted phenol with formaldehyde. Used as the basis for Bakelite, PFs were the first commercial synthetic resins (plastics). They have been widely used for the production of molded products including billiard balls, laboratory countertops, and as coatings and adhesives. They were at one time the primary material used for the production of circuit boards but have been largely replaced with epoxy resins and fiberglass cloth, as with fire-resistant FR-4 circuit board materials.
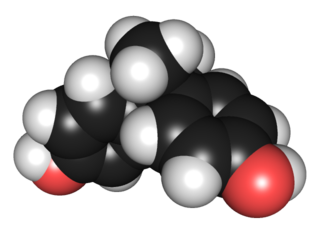
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes.
Pre-preg is a composite material made from "pre-impregnated" fibers and a partially cured polymer matrix, such as epoxy or phenolic resin, or even thermoplastic mixed with liquid rubbers or resins. The fibers often take the form of a weave and the matrix is used to bond them together and to other components during manufacture. The thermoset matrix is only partially cured to allow easy handling; this B-Stage material requires cold storage to prevent complete curing. B-Stage pre-preg is always stored in cooled areas since heat accelerates complete polymerization. Hence, composite structures built of pre-pregs will mostly require an oven or autoclave to cure. The main idea behind a pre-preg material is the use of anisotropic mechanical properties along the fibers, while the polymer matrix provides filling properties, keeping the fibers in a single system.

Powder coating is a type of coating that is applied as a free-flowing, dry powder. Unlike conventional liquid paint, which is delivered via an evaporating solvent, powder coating is typically applied electrostatically and then cured under heat or with ultraviolet light. The powder may be a thermoplastic or a thermoset polymer. It is usually used to create a thick, tough finish that is more durable than conventional paint. Powder coating is mainly used for coating of metal objects, particularly those subject to rough use. Advancements in powder coating technology like UV-curable powder coatings allow for other materials such as plastics, composites, carbon fiber, and MDF to be powder coated, as little heat or oven dwell time is required to process them.
Vinyl ester resin, or often just vinyl ester, is a resin produced by the esterification of an epoxy resin with acrylic or methacrylic acids. The "vinyl" groups refer to these ester substituents, which are prone to polymerize and thus an inhibitor is usually added. The diester product is then dissolved in a reactive solvent, such as styrene, to approximately 35–45 percent content by weight. Polymerization is initiated by free radicals, which are generated by UV-irradiation or peroxides.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
Synthetic resins are industrially produced resins, typically viscous substances that convert into rigid polymers by the process of curing. In order to undergo curing, resins typically contain reactive end groups, such as acrylates or epoxides. Some synthetic resins have properties similar to natural plant resins, but many do not.
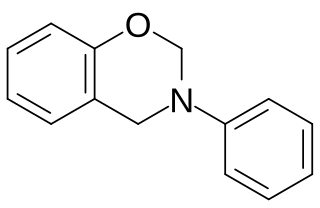
Polybenzoxazines, also called benzoxazine resins, are cured polymerization products derived from benzoxazine monomers.
Polyester resins are synthetic resins formed by the reaction of dibasic organic acids and polyhydric alcohols. Maleic anhydride is a commonly used raw material with diacid functionality in unsaturated polyester resins. Unsaturated polyester resins are used in sheet moulding compound, bulk moulding compound and the toner of laser printers. Wall panels fabricated from polyester resins reinforced with fiberglass—so-called fiberglass reinforced plastic (FRP)—are typically used in restaurants, kitchens, restrooms and other areas that require washable low-maintenance walls. They are also used extensively in cured-in-place pipe applications. Departments of Transportation in the USA also specify them for use as overlays on roads and bridges. In this application they are known AS Polyester Concrete Overlays (PCO). These are usually based on isophthalic acid and cut with styrene at high levels—usually up to 50%. Polyesters are also used in anchor bolt adhesives though epoxy based materials are also used. Many companies have and continue to introduce styrene free systems mainly due to odor issues, but also over concerns that styrene is a potential carcinogen. Drinking water applications also prefer styrene free. Most polyester resins are viscous, pale coloured liquids consisting of a solution of a polyester in a reactive diluent which is usually styrene, but can also include vinyl toluene and various acrylates.
Bulk moulding compound (BMC), bulk moulding composite, or dough moulding compound (DMC), is a ready-to-mold, glass-fiber reinforced thermoset polymer material primarily used in compression moulding, as well as in injection moulding and transfer moulding. Typical applications include demanding electrical applications, corrosion resistant needs, appliance, automotive, and transit.
Sheet moulding compound (SMC) or sheet moulding composite is a ready to mould glass-fibre reinforced polyester material primarily used in compression moulding. The sheet is provided in rolls weighing up to 1000 kg. Alternatively the resin and related materials may be mixed on site when a producer wants greater control over the chemistry and filler.
BT-Epoxy is one of a number of thermoset resins used in printed circuit boards (PCBs). It is a mixture of epoxy resin, a common raw material for PCBs and BT resins. This is in turn a mixture of bismaleimide, which is also used as a raw material for PCBs and cyanate ester. Three cyano groups of the cyanate ester are trimerized to a triazine ring structure, hence the T in the name. In presence of a bismaleimide, the double bond of the maleimide group can copolymerize with the cyano groups to heterocyclic 6-membered aromatic ring structures with two nitrogen atoms (pyrimidines). The cure reaction occurs at temperatures up to 250 °C (482 °F), and is catalyzed by strongly basic molecules like Dabco (diazabicyclooctane) and 4-DMAP (4-dimethylaminopyridin). Products with very high glass transition temperatures (Tg) up to 300 °C (572 °F) and very low dielectric constant can be obtained. These properties make these materials very attractive for use in PCBs, which are often subjected to such conditions.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle.

Bisphenol A diglycidyl ether is an organic compound and is a liquid epoxy resin. The compound is a colorless viscous liquid. It is a key component of many epoxy resin formulations. Addition of further Bisphenol A and a catalyst and heat can produce Bisphenol A glycidyl ether epoxy resins of higher molecular weight that are solid.
Vitrimers are a class of plastics, which are derived from thermosetting polymers (thermosets) and are very similar to them. Vitrimers consist of molecular, covalent networks, which can change their topology by thermally activated bond-exchange reactions. At high temperatures they can flow like viscoelastic liquids, at low temperatures the bond-exchange reactions are immeasurably slow (frozen) and the Vitrimers behave like classical thermosets at this point. Vitrimers are strong glass formers. Their behavior opens new possibilities in the application of thermosets like as a self-healing material or simple processibility in a wide temperature range.
In materials science, a polymer matrix composite (PMC) is a composite material composed of a variety of short or continuous fibers bound together by a matrix of organic polymers. PMCs are designed to transfer loads between fibers of a matrix. Some of the advantages with PMCs include their light weight, high resistance to abrasion and corrosion, and high stiffness and strength along the direction of their reinforcements.
In materials science, a matrix is a constituent of a composite material.










