Contents
- History
- Production
- Properties
- Uses and applications
- Main uses
- Minor uses
- BPA substitutes
- Human safety
- Exposure
- Health effects and regulation
- Pharmacology
- Environmental safety
- Distribution and degradation
- Environmental effects
- See also
- References
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 4,4′-(Propane-2,2-diyl)diphenol | |
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.133 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2430 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C15H16O2 | |
| Molar mass | 228.291 g·mol−1 |
| Appearance | White solid |
| Odor | Phenolic, medical |
| Density | 1.217 g/cm3 [1] |
| Melting point | 155 °C (311 °F; 428 K) [2] |
| Boiling point | 250–252 °C (482–486 °F; 523–525 K) [2] at 13 torrs (0.017 atm) |
| 0.3 g/L (25 °C) [3] | |
| log P | 3.41 [4] |
| Vapor pressure | 5×10−6 Pa (25 °C) [5] |
| Hazards [6] | |
| GHS labelling: | |
    | |
| Danger | |
| H317, H318, H335, H360, H411 [6] | |
| P201, P202, P261, P273, P302+P352, P304+P340, P305+P351+P338, P308+P313, P333+P313, P363, P403+P233 [6] | |
| NFPA 704 (fire diamond) | |
| Flash point | 227 °C (441 °F; 500 K) [6] |
| 510 °C (950 °F; 783 K) [6] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
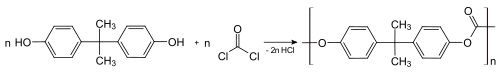
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. [3] [7] BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes. [8]
BPA's largest single application is as a co-monomer in the production of polycarbonates, which accounts for 65–70% of all BPA production. [9] [10] The manufacturing of epoxy resins and vinyl ester resins account for 25–30% of BPA use. [9] [10] The remaining 5% is used as a major component of several high-performance plastics, and as a minor additive in polyvinyl chloride (PVC), polyurethane, thermal paper, and several other materials. It is not a plasticizer, [11] although it is often wrongly labelled as such.
The health effects of BPA have been the subject of prolonged public and scientific debate. [12] [13] [14] BPA is a xenoestrogen, exhibiting hormone-like properties that mimic the effects of estrogen in the body. [15] Although the effect is very weak, [16] the pervasiveness of BPA-containing materials raises concerns, as exposure is effectively lifelong. Many BPA-containing materials are non-obvious but commonly encountered, [17] and include coatings for the inside of food cans, [18] clothing designs, [19] shop receipts, [20] and dental fillings. [21] BPA has been investigated by public health agencies in many countries, as well as by the World Health Organization. [12]
While normal exposure is below the level currently associated with risk, several jurisdictions have taken steps to reduce exposure on a precautionary basis, in particular by banning BPA from baby bottles. There is some evidence that BPA exposure in infants has decreased as a result of this. [22] BPA-free plastics have also been introduced, which are manufactured using alternative bisphenols such as bisphenol S and bisphenol F, but there is also controversy around whether these are actually safer. [23] [24] [25]