| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2-(Chloromethyl)oxirane | |||
| Other names (Chloromethyl)oxirane Epichlorohydrin 1-Chloro-2,3-epoxypropane γ-Chloropropylene oxide Glycidyl chloride ECH | |||
| Identifiers | |||
3D model (JSmol) | |||
| 79785 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.128 | ||
| EC Number |
| ||
| 164180 | |||
| KEGG | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 2023 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C3H5ClO | |||
| Molar mass | 92.52 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | garlic or chloroform-like | ||
| Density | 1.1812 g/cm3 | ||
| Melting point | −25.6 °C (−14.1 °F; 247.6 K) | ||
| Boiling point | 117.9 °C (244.2 °F; 391.0 K) | ||
| 7% (20°C) [2] | |||
| Vapor pressure | 13 mmHg (20°C) [2] | ||
| Hazards | |||
| GHS labelling: | |||
     | |||
| Danger | |||
| H226, H301, H311, H314, H317, H331, H350 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P272, P280, P281, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P308+P313, P310, P311, P312, P321, P322, P330, P333+P313, P361, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 32 °C (90 °F; 305 K) | ||
| Explosive limits | 3.8–21% [2] | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) | 3617 ppm (rat, 1 hr) 2165 ppm (rat, 1 hr) 250 ppm (rat, 8 hr) 244 ppm (rat, 8 hr) 360 ppm (rat, 6 hr) [3] | ||
LCLo (lowest published) | 250 ppm (rat, 4 hr) [3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | TWA 5 ppm (19 mg/m3) [skin] [2] | ||
REL (Recommended) | Carcinogen [2] | ||
IDLH (Immediate danger) | Ca [75 ppm] [2] | ||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
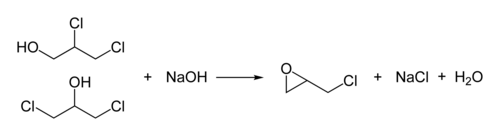
Epichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents. [4] It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive electrophilic compound and is used in the production of glycerol, plastics, epoxy glues and resins, epoxy diluents and elastomers.