Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.

Megestrol acetate (MGA), sold under the brand name Megace among others, is a progestin medication which is used mainly as an appetite stimulant to treat wasting syndromes such as cachexia. It is also used to treat breast cancer and endometrial cancer, and has been used in birth control. Megestrol acetate is generally formulated alone, although it has been combined with estrogens in birth control formulations. It is usually taken by mouth.

Gestonorone caproate, also known as gestronol hexanoate or norhydroxyprogesterone caproate and sold under the brand names Depostat and Primostat, is a progestin medication which is used in the treatment of enlarged prostate and cancer of the endometrium. It is given by injection into muscle typically once a week.
Feminizing hormone therapy, also known as transfeminine hormone therapy, is hormone therapy and sex reassignment therapy to change the secondary sex characteristics of transgender people from masculine or androgynous to feminine. It is a common type of transgender hormone therapy and is used to treat transgender women and non-binary transfeminine individuals. Some, in particular intersex people, but also some non-transgender people, take this form of therapy according to their personal needs and preferences.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.
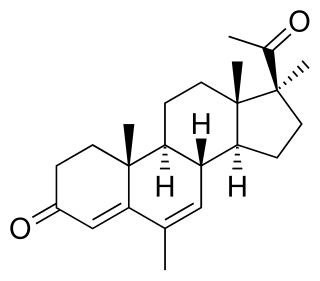
Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. It is taken by mouth.

Allylestrenol, sold under the brand names Gestanin and Turinal among others, is a progestin medication which is used to treat recurrent and threatened miscarriage and to prevent premature labor in pregnant women. However, except in the case of proven progesterone deficiency, its use for such purposes is no longer recommended. It is also used in Japan to treat benign prostatic hyperplasia (BPH) in men. The medication is used alone and is not formulated in combination with an estrogen. It is taken by mouth.

Nomegestrol acetate (NOMAC), sold under the brand names Lutenyl and Zoely among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. NOMAC is taken by mouth. A birth control implant for placement under the skin was also developed but ultimately was not marketed.

Cyproterone acetate (CPA), sold alone under the brand name Androcur or with ethinylestradiol under the brand names Diane or Diane-35 among others, is an antiandrogen and progestin medication used in the treatment of androgen-dependent conditions such as acne, excessive body hair growth, early puberty, and prostate cancer, as a component of feminizing hormone therapy for transgender individuals, and in birth control pills. It is formulated and used both alone and in combination with an estrogen. CPA is taken by mouth one to three times per day.

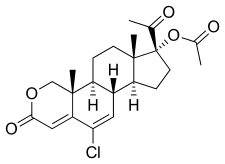
Cyproterone, also known by its developmental code name SH-80881, is a steroidal antiandrogen which was studied in the 1960s and 1970s but was never introduced for medical use. It is a precursor of cyproterone acetate (CPA), an antiandrogen, progestin, and antigonadotropin which was introduced instead of cyproterone and is widely used as a medication. Cyproterone and CPA were among the first antiandrogens to be developed.

Delmadinone acetate (DMA), sold under the brand name Tardak among others, is a progestin and antiandrogen which is used in veterinary medicine to treat androgen-dependent conditions such as benign prostatic hyperplasia. It must be used with care as it has the potential to cause adrenal insufficiency via inhibition of adrenocorticotropic hormone (ACTH) secretion from the pituitary gland. DMA is the C17α acetate ester of delmadinone, which, in contrast to DMA, was never marketed for medical use.

Benorterone, also known by its developmental code name SKF-7690 and as 17α-methyl-B-nortestosterone, is a steroidal antiandrogen which was studied for potential medical use but was never marketed. It was the first known antiandrogen to be studied in humans. It is taken by mouth or by application to skin.

Oxendolone, sold under the brand names Prostetin and Roxenone, is an antiandrogen and progestin medication which is used in Japan in the treatment of enlarged prostate. However, this use is controversial due to concerns about its clinical efficacy. Oxendolone is not effective by mouth and must be given by injection into muscle.
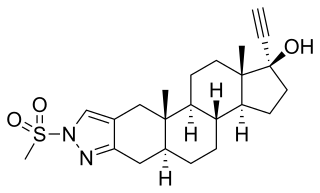
Zanoterone, also known as (5α,17α)-1'-(methylsulfonyl)-1'-H-pregn-20-yno[3,2-c]pyrazol-17-ol, is a steroidal antiandrogen which was never marketed. It was investigated for the treatment of benign prostatic hyperplasia (BPH) but failed to demonstrate sufficient efficacy in phase II clinical trials, and also showed an unacceptable incidence rate and severity of side effects. As such, it was not further developed.

Hydroxyprogesterone acetate (OHPA), sold under the brand name Prodox, is an orally active progestin related to hydroxyprogesterone caproate (OHPC) which has been used in clinical and veterinary medicine. It has reportedly also been used in birth control pills.

Edogestrone, or edogesterone, also known as 17α-acetoxy-3,3-ethylenedioxy-6-methylpregn-5-en-20-one, is a steroidal progestin and antiandrogen of the 17α-hydroxyprogesterone group which was synthesized in 1964 but was never marketed. Similarly to the structurally related steroid cyproterone acetate, edogestrone binds directly to the androgen receptor and antagonizes it, displacing androgens like testosterone from the receptor, though not as potently as cyproterone acetate. The drug has also been found to suppress androgen production, likely via progesterone receptor activation-mediated antigonadotropic activity.

A steroidal antiandrogen (SAA) is an antiandrogen with a steroidal chemical structure. They are typically antagonists of the androgen receptor (AR) and act both by blocking the effects of androgens like testosterone and dihydrotestosterone (DHT) and by suppressing gonadal androgen production. SAAs lower concentrations of testosterone through simulation of the negative feedback inhibition of the hypothalamus. SAAs are used in the treatment of androgen-dependent conditions in men and women, and are also used in veterinary medicine for the same purpose. They are the converse of nonsteroidal antiandrogens (NSAAs), which are antiandrogens that are not steroids and are structurally unrelated to testosterone.

17α-Allyl-19-nortestosterone, also known as 3-ketoallylestrenol or as 17α-allylestr-4-en-17β-ol-3-one, is a progestin which was never marketed. It is a combined derivative of the anabolic–androgenic steroid and progestogen nandrolone (19-nortestosterone) and the antiandrogen allyltestosterone (17α-allyltestosterone). The drug is a major active metabolite of allylestrenol, which is thought to be a prodrug of 17α-allyl-19-nortestosterone.

Cymegesolate, also known as cypionyl megestrol acetate or as megestrol acetate 3β-cypionate, is a progestin medication which was never marketed. It was developed in China in the late 1970s and early to mid 1980s for use as a hormonal contraceptive. The medication was formulated at a dose of 50–100 mg in combination with a "trace" dose of 0.25–0.5 mg quinestrol as a long-lasting, once-a-month combined oral contraceptive pill. This combination has been studied in 1,213 women across a total of 9,651 menstrual cycles, with contraceptive effectiveness of over 99.13% and "very few side effects." At the high dose, it showed an anovulation rate of only about 60%, and instead mediated its contraceptive effects via a marked anti-implantation effect.

The pharmacology of cyproterone acetate (CPA) concerns the pharmacology of the steroidal antiandrogen and progestin medication cyproterone acetate.