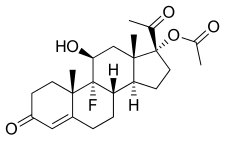
Fluorometholone, also known as 6α-methyl-9α-fluoro-11β,17α-dihydroxypregna-1,4-diene-3,20-dione, is a synthetic glucocorticoid which is used in the treatment of inflammatory eye diseases. The C17α acetate ester, fluorometholone acetate, is also a glucocorticoid and is used for similar indications.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Dimethisterone, formerly sold under the brand names Lutagan and Secrosteron among others, is a progestin medication which was used in birth control pills and in the treatment of gynecological disorders but is now no longer available. It was used both alone and in combination with an estrogen. It is taken by mouth.

Melengestrol acetate (MLGA), sold under the brand names Heifermax and MGA among others, is a progestin medication which is used in animal reproduction. It is not approved for use in humans, and is instead used as an implantable contraceptive for captive animals in zoos and other refuges, and is also used as a feed additive to promote growth in cattle, a purpose it is licensed for in the United States and Canada.

Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. It is taken by mouth.

Delmadinone acetate (DMA), sold under the brand name Tardak among others, is a progestin and antiandrogen which is used in veterinary medicine to treat androgen-dependent conditions such as benign prostatic hyperplasia. It must be used with care as it has the potential to cause adrenal insufficiency via inhibition of adrenocorticotropic hormone (ACTH) secretion from the pituitary gland. DMA is the C17α acetate ester of delmadinone, which, in contrast to DMA, was never marketed for medical use.

Osaterone acetate, sold under the brand name Ypozane, is a medication which is used in veterinary medicine in Europe in the treatment of enlarged prostate in dogs. It is given by mouth.

Hydroxyprogesterone acetate (OHPA), sold under the brand name Prodox, is an orally active progestin related to hydroxyprogesterone caproate (OHPC) which has been used in clinical and veterinary medicine. It has reportedly also been used in birth control pills.
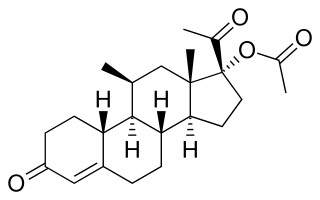
Norgestomet, or norgestamet, sold under the brand name Syncro-Mate B and Crestar, is a progestin medication which is used in veterinary medicine to control estrus and ovulation in cattle.

Anagestone acetate, sold under the brand names Anatropin and Neo-Novum, is a progestin medication which was withdrawn from medical use due to carcinogenicity observed in animal studies.

Ethynerone, also known as 17α-(2-chloroethynyl)estra-4,9-dien-17β-ol-3-one, is a steroidal progestin of the 19-nortestosterone group that was first reported in 1961 but was never marketed. Under the developmental code name MK-665, it was studied in combination with mestranol as an oral contraceptive. Development of the drug was discontinued due to concerns surrounding toxicity findings in dogs. It is a chloroethynylated derivative of norethisterone.

Flugestone, also known as flurogestone, as well as 9α-fluoro-11β,17α-dihydroxyprogesterone, is a steroidal progestin of the 17α-hydroxyprogesterone group that was never marketed. An acetate ester, flurogestone acetate, is used in veterinary medicine.

Pentagestrone acetate (PGA), sold under the brand names Gestovis and Gestovister, is a progestin which was described in the literature in 1960 and was introduced by Vister in Italy in 1961. It is the 3-cyclopentyl enol ether of 17α-hydroxyprogesterone acetate. PGA, along with quingestrone, is said to have very similar properties to those of dydrogesterone, a pure progestogen and close analogue of progesterone.

Edogestrone, or edogesterone, also known as 17α-acetoxy-3,3-ethylenedioxy-6-methylpregn-5-en-20-one, is a steroidal progestin and antiandrogen of the 17α-hydroxyprogesterone group which was synthesized in 1964 but was never marketed. Similarly to the structurally related steroid cyproterone acetate, edogestrone binds directly to the androgen receptor and antagonizes it, displacing androgens like testosterone from the receptor, though not as potently as cyproterone acetate. The drug has also been found to suppress androgen production, likely via progesterone receptor activation-mediated antigonadotropic activity.

Acetomepregenol (ACM), also known as mepregenol diacetate and sold under the brand name Diamol, is a progestin medication which is used in Russia for the treatment of gynecological conditions and as a method of birth control in combination with an estrogen. It has also been studied in the treatment of threatened abortion. It has been used in veterinary medicine as well. It has been marketed since at least 1981.
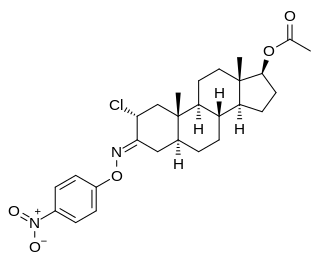
Nisterime acetate (USAN) (developmental code name ORF-9326), also known as 2α-chloro-4,5α-dihydrotestosterone O-(p-nitrophenyl)oxime 17β-acetate or as 2α-chloro-5α-androstan-17β-ol-3-one O-(p-nitrophenyl)oxime 17β-acetate, is a synthetic, orally active anabolic-androgenic steroid (AAS) and a derivative of dihydrotestosterone (DHT) that was developed as a postcoital contraceptive but was never marketed. It is an androgen ester – specifically, the C17α acetate ester of nisterime. Unlike antiprogestogens like mifepristone, nisterime acetate does not prevent implantation and instead induces embryo resorption as well as interrupts the post-implantation stage of pregnancy.

11-Ketoprogesterone, or 11-oxoprogesterone, also known as pregn-4-ene-3,11,20-trione, is a pregnane steroid related to cortisone (11-keto-17α,21-dihydroxyprogesterone) that was formerly used in veterinary medicine in the treatment of bovine ketosis. It was synthesized in 1940. The steroid has profound effects on carbohydrate metabolism and possesses activities associated with adrenal cortex hormones like cortisone. However, it is non-toxic even in high dosages, suggesting that it lacks conventional glucocorticoid activity, and it does not possess mineralocorticoid activity, unlike other adrenocortical hormones. 11-Ketoprogesterone may act through membrane glucocorticoid receptors.
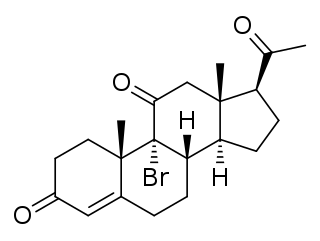
Bromoketoprogesterone (BKP), also known as 9α-bromo-11-oxoprogesterone (BOP), and known by the tentative brand name Braxarone (Squibb), is an orally active progestin which does not appear to have been marketed.

Megestrol caproate, abbreviated as MGC, is a progestin medication which was never marketed. It was developed in Russia in 2002. In animals, MGC shows 10-fold higher progestogenic activity compared to progesterone when both are administered via subcutaneous injection. In addition, MGC has no androgenic, anabolic, or estrogenic activity. The medication was suggested as a potential contraceptive and therapeutic agent.