 | |
| Clinical data | |
|---|---|
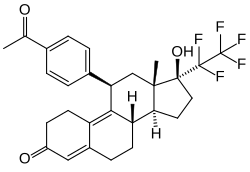
| Other names | ZK-230211; BAY 86-5044; ZK-PRA; 11β-(4-acetylphenyl)-17β-hydroxy-17α-(1,1,2,2,2-pentafluoroethyl)estra-4,9-dien-3-one |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.190.674 |
| Chemical and physical data | |
| Formula | C28H29F5O3 |
| Molar mass | 508.529 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lonaprisan (INN, USAN) (developmental code names ZK-230211, BAY 86-5044, ZK-PRA) is a synthetic, steroidal antiprogestogen which was under development by Bayer HealthCare Pharmaceuticals for the treatment of endometriosis, dysmenorrhea, and breast cancer but was discontinued. [1] [2] [3] It is a potent and highly selective silent antagonist of the progesterone receptor (PR). [2] [3] [4] The drug reached phase II clinical trials prior to its discontinuation. [1]