
Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.

A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.
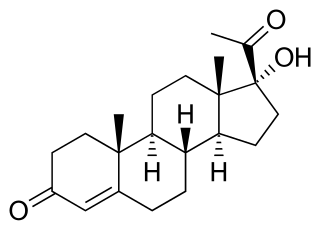
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.

Gestodene, sold under the brand names Femodene and Minulet among others, is a progestin medication which is used in birth control pills for women. It is also used in menopausal hormone therapy. The medication is available almost exclusively in combination with an estrogen. It is taken by mouth.

Gestonorone caproate, also known as gestronol hexanoate or norhydroxyprogesterone caproate and sold under the brand names Depostat and Primostat, is a progestin medication which is used in the treatment of enlarged prostate and cancer of the endometrium. It is given by injection into muscle typically once a week.
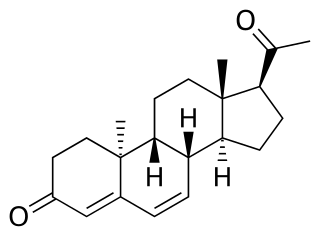
Dydrogesterone, sold under the brand name Duphaston among others, is a progestin medication which is used for a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy. It is taken by mouth.

Norgestrienone, sold under the brand names Ogyline, Planor, and Miniplanor, is a progestin medication which has been used in birth control pills, sometimes in combination with ethinylestradiol. It was developed by Roussel Uclaf and has been registered for use only in France. Under the brand name Planor, it has been marketed in France as 2 mg norgestrienone and 50 μg ethinylestradiol tablets. It is taken by mouth.

Algestone acetophenide, also known more commonly as dihydroxyprogesterone acetophenide (DHPA) and sold under the brand names Perlutal and Topasel among others, is a progestin medication which is used in combination with an estrogen as a form of long-lasting injectable birth control. It has also been used alone, but is no longer available as a standalone medication. DHPA is not active by mouth and is given once a month by injection into muscle.
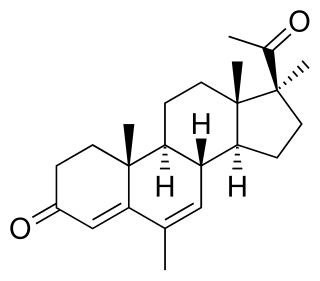
Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. It is taken by mouth.
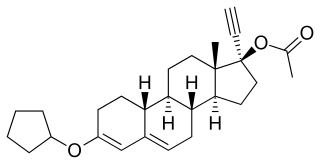
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.
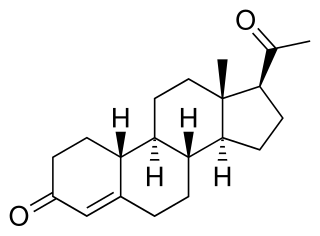
19-Norprogesterone, also known as 19-norpregn-4-ene-3,20-dione, is a steroidal progestin and close analogue of the sex hormone progesterone, lacking only the C19 methyl group of that molecule. It was first synthesized in 1944 in the form of a mixture that also included unnatural stereoisomers of progesterone, and this mixture was found to be at least equivalent to progesterone in terms of progestogenic activity. Subsequent investigations revealed that 17-isoprogesterone and 14-iso-17-isoprogesterone are devoid of progestogenic activity. 19-Norprogesterone was resynthesized in 1951 with an improved method, and was confirmed to be the component of the mixture synthesized in 1944 that was responsible for its progestogenic activity. In 1953, a paper was published showing that 19-norprogesterone possessed 4- to 8-fold the activity of progesterone in the Clauberg assay in rabbits, and at the time of this discovery, 19-norprogesterone was the most potent progestogen known.
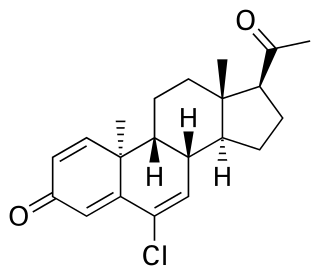
Trengestone, sold under the brand names Reteroid, Retroid, and Retrone, is a progestin medication which was formerly used to treat menstrual disorders but is now no longer marketed. It is taken by mouth.

Hydroxyprogesterone acetate (OHPA), sold under the brand name Prodox, is an orally active progestin related to hydroxyprogesterone caproate (OHPC) which has been used in clinical and veterinary medicine. It has reportedly also been used in birth control pills.

Hydroxyprogesterone heptanoate (OHPH), also known as hydroxyprogesterone enanthate (OHPE) and sold under the brand names H.O.P., Lutogil A.P., and Lutogyl A.P. among others, is a progestin medication used for progestogenic indications. It has been formulated both alone and in together with estrogens, androgens/anabolic steroids, and other progestogens in several combination preparations. OHPH is given by injection into muscle at regular intervals.

Oxogestone phenpropionate, also known as xinogestone, as well as 20β-hydroxy-19-norprogesterone 20β-(3-phenylpropionate), is a progestin related to the 19-norprogesterone derivatives which was developed as an injectable hormonal contraceptive, specifically a progestogen-only injectable contraceptive, in the 1960s and early 1970s but was never marketed. It was studied at a dose of 50 to 75 mg once a month by intramuscular injection but was associated with a high failure rate with this regimen and was not further developed. OPP is the 20β-(3-phenylpropionate) ester of oxogestone, which, similarly, was never marketed.

Ro 6-3129, also known as 16α-ethylthio-6-dehydroretroprogesterone or as 16α-ethylthio-9β,10α-pregna-4,6-diene-3,20-dione, as well as 16α-ethylthiodydrogesterone, is a progestogen of the retroprogesterone group which was developed by Roche but was never marketed. It shows greater potency than dydrogesterone in bioassays.

DU-41164, also known as 1,2β-methylene-6-fluoro-17α-acetoxy-δ6-retroprogesterone, is a progestin which was developed by Philips-Duphar in the 1970s and was never marketed. It is a combined derivative of 17α-hydroxyprogesterone and retroprogesterone. The drug shows extremely high potency as a progestogen in animals; it was reported to possess 500 times the affinity of progesterone for the progesterone receptor expressed in rabbit uterus, and showed 600 times the progestogenic potency of subcutaneous progesterone when given orally in animals. The affinity of DU-41164 for the progesterone receptor was described in 1974 as "probably the highest reported for any steroid-receptor interaction". The drug showed no androgenic, anabolic, antiandrogenic, estrogenic, or corticosteroid activity in animals. Although highly potent in animals, DU-41164 produced little or no progestogenic effect at dosages of 50 and 200 µg/day in women, suggesting major species differences. A closely related compound, DU-41165, has been developed as a photoaffinity label for the progesterone receptor.
The pharmacology of progesterone, a progestogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.

Lynestrenol phenylpropionate (LPP), also known as ethynylestrenol phenylpropionate, is a progestin and a progestogen ester which was developed for potential use as a progestogen-only injectable contraceptive by Organon but was never marketed. It was assessed at doses of 25 to 75 mg in an oil solution once a month by intramuscular injection. LPP was associated with high contraceptive failure at the low dose and with poor cycle control. The medication was found to produce estrogenic effects in the endometrium in women due to transformation into estrogenic metabolites.


















