
Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the major progestogen in the body. Progesterone has a variety of important functions in the body. It is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid.
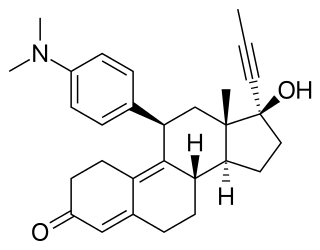
Mifepristone, also known as RU-486, is a medication typically used in combination with misoprostol to bring about a medical abortion during pregnancy and manage early miscarriage. This combination is 97% effective during the first 63 days of pregnancy. It is also effective in the second trimester of pregnancy. It is taken by mouth.

A freemartin or free-martin is an infertile female cattle with masculinized behavior and non-functioning ovaries. Phenotypically, the animal appears female, but various aspects of female reproductive development are altered due to acquisition of anti-Müllerian hormone from the male twin. Genetically, the animal is chimeric: karyotypy of a sample of cells shows XX/XY chromosomes. The animal originates as a female (XX), but acquires the male (XY) component in utero by exchange of some cellular material from a male twin, via vascular connections between placentas: an example of microchimerism. The chimerism is mainly present in the hematopoietic stem cells.

Semen collection refers to the process of obtaining semen from human males or other animals with the use of various methods, for the purposes of artificial insemination, or medical study. Semen can be collected via masturbation, prostate massage, artificial vagina, penile vibratory stimulation (vibroejaculation) and electroejaculation. Semen can be collected from endangered species for cryopreservation of genetic resources.
The estrous cycle is a set of recurring physiological changes induced by reproductive hormones in females of mammalian subclass Theria. Estrous cycles start after sexual maturity in females and are interrupted by anestrous phases, otherwise known as "rest" phases, or by pregnancies. Typically, estrous cycles repeat until death. These cycles are widely variable in duration and frequency depending on the species. Some animals may display bloody vaginal discharge, often mistaken for menstruation. Many mammals used in commercial agriculture, such as cattle and sheep, may have their estrous cycles artificially controlled with hormonal medications for optimum productivity. The male equivalent, seen primarily in ruminants, is called rut.
Relaxin is a protein hormone of about 6000 Da, first described in 1926 by Frederick Hisaw.
The dog is a domesticated descendant of the wolf. Also called the domestic dog, it is derived from the extinct Pleistocene wolf; the gray wolf is the dog's closest living relative. The dog was the first species to be domesticated by humans. Experts estimate that hunter-gatherers domesticated dogs more than 15,000 years ago, which was before the development of agriculture. Due to their long association with humans, dogs have expanded to a large number of domestic individuals and gained the ability to thrive on a starch-rich diet that would be inadequate for other canids.
Canine reproduction is the process of sexual reproduction in domestic dogs, wolves, coyotes and other canine species.

Trilostane, sold under the brand name Vetoryl among others, is a medication which has been used in the treatment of Cushing's syndrome, Conn's syndrome, and postmenopausal breast cancer in humans. It was withdrawn for use in humans in the United States in the 1990s but was subsequently approved for use in veterinary medicine in the 2000s to treat Cushing's syndrome in dogs. It is taken by mouth.
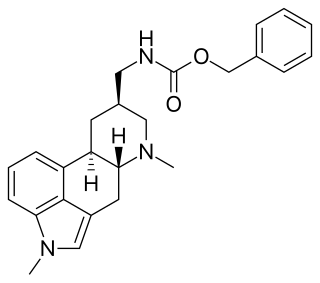
Metergoline, also known as methergoline and sold under the brand names Contralac (veterinary) and Liserdol (clinical), is a monoaminergic medication of the ergoline group which is used as a prolactin inhibitor in the treatment of hyperprolactinemia and to suppress lactation.

Nesting behavior refers to an instinct in animals during reproduction to prepare a place with optimal conditions for offspring. The nesting place provides protection against predators and competitors that mean to exploit or kill offspring. It also provides protection against the physical environment.
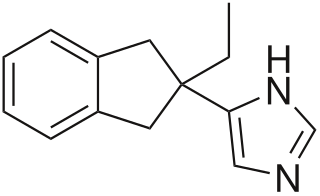
Atipamezole, sold under the brand name Antisedan among others, is a synthetic α2 adrenergic receptor antagonist used for the reversal of the sedative and analgesic effects of dexmedetomidine and medetomidine in dogs. Its reversal effect works by competing with the sedative for α2-adrenergic receptors and displacing them. It is mainly used in veterinary medicine, and while it is only licensed for dogs and for intramuscular use, it has been used intravenously, as well as in cats and other animals(intravenous use in cats and dogs is not recommended due to the potential for cardiovascular collapse. This occurs due to profound hypotension caused by reversal of the alpha 1 effects while the reflex bradycardia is still in effect.). There is a low rate of side effects, largely due to atipamezole's high specificity for the α2-adrenergic receptor. Atipamezole has a very quick onset, usually waking an animal up within 5 to 10 minutes.

Altrenogest, sold under the brand names Swinemate and Altren manufactured by Aurora Pharmaceutical and Regumate manufactured by Merck, is a progestin of the 19-nortestosterone group which is widely used in veterinary medicine to suppress or synchronize estrus in horses and pigs. It is available for veterinary use in both Europe and the United States.

Almost all mammal penises have foreskins or prepuces, although in non-human cases, the foreskin is usually a sheath into which the whole penis is retracted. In koalas, the foreskin contains naturally occurring bacteria that play an important role in fertilization. In some bat species, the prepuce contains an erectile tissue structure called the accessory corpus cavernosus.

Osaterone acetate, sold under the brand name Ypozane, is a medication which is used in veterinary medicine in Europe in the treatment of enlarged prostate in dogs. It is given by mouth.

5β-Dihydroprogesterone is an endogenous neurosteroid and an intermediate in the biosynthesis of pregnanolone and epipregnanolone from progesterone. It is synthesized from progesterone by the enzyme 5β-reductase.

Toripristone is a synthetic, steroidal antiglucocorticoid as well as antiprogestogen which was never marketed. It is reported as a potent and highly selective antagonist of the glucocorticoid receptor (GR), though it also acts as an antagonist of the progesterone receptor (PR). The pharmacological profile of toripristone is said to be very similar to that of mifepristone, except that toripristone does not bind to orosomucoid. The drug has been used to study the hypothalamic-pituitary-adrenal axis and has been used as a radiotracer for the GR. Its INN was given in 1990.

Lilopristone (INN) is a synthetic, steroidal antiprogestogen with additional antiglucocorticoid activity which was developed by Schering and was patented in 1985. It is described as an abortifacient and endometrial contraceptive. The drug differs from mifepristone only in the structure of its C17α side chain, and is said to have much reduced antiglucocorticoid activity in comparison.
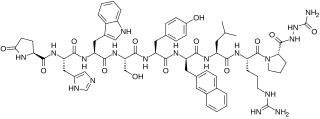
Azagly-nafarelin, sold under the brand name Gonazon, is a gonadotropin-releasing hormone agonist medication which is used in veterinary medicine in Europe. It is a GnRH analogue and a synthetic peptide, specifically a decapeptide. The medication has been approved in Europe as a solid silicone-based matrix implant for use as a contraceptive in animals such as male dogs, cats, and others, but is no longer or was never commercially available. The medication has also been used to treat benign prostatic hyperplasia in animals. In addition to its use in mammals, azagly-nafarelin has been approved for use in aquaculture fish, specifically to control ovulation in salmonids, and was the first GnRH agonist to be available for use in fish. It was introduced for use by 2005.
Non-surgical fertility control is the prevention of reproduction without the use of surgery. The most common form of sterilization in dogs and cats is surgical, spaying in females and castration in males. Non-surgical fertility control can either result in sterilization or temporary contraception and could offer a cheaper way to keep wild dog and cat populations under control. As of 2019, only contraceptives are commercially available. Research is ongoing into methods that could result in permanent suppression of fertility.
















