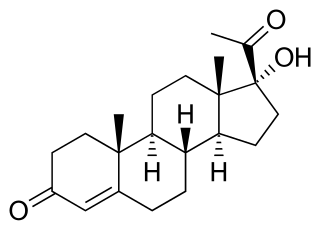
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.

Medroxyprogesterone (MP), is a progestin which is not used medically. A derivative, medroxyprogesterone acetate (MPA), is used as a medication in humans, and is far more widely known in comparison. Medroxyprogesterone is sometimes used as a synonym for medroxyprogesterone acetate, and what is almost always being referred to when the term is used is MPA and not medroxyprogesterone.
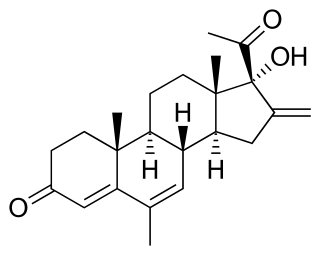
Melengestrol is a steroidal progestin of the 17α-hydroxyprogesterone group and an antineoplastic drug which was never marketed. An acylated derivative, melengestrol acetate, is used as a growth promoter in animals.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. It is taken by mouth.

Delmadinone acetate (DMA), sold under the brand name Tardak among others, is a progestin and antiandrogen which is used in veterinary medicine to treat androgen-dependent conditions such as benign prostatic hyperplasia. It must be used with care as it has the potential to cause adrenal insufficiency via inhibition of adrenocorticotropic hormone (ACTH) secretion from the pituitary gland. DMA is the C17α acetate ester of delmadinone, which, in contrast to DMA, was never marketed for medical use.

Segesterone acetate (SGA), sold under the brand names Nestorone, Elcometrine, and Annovera, is a progestin medication which is used in birth control and in the treatment of endometriosis in the United States, Brazil, and other South American countries. It is available both alone and in combination with an estrogen. It is not effective by mouth and must be given by other routes, most typically as a vaginal ring or implant that is placed into fat.

Osaterone acetate, sold under the brand name Ypozane, is a medication which is used in veterinary medicine in Europe in the treatment of enlarged prostate in dogs. It is given by mouth.

Hydroxyprogesterone acetate (OHPA), sold under the brand name Prodox, is an orally active progestin related to hydroxyprogesterone caproate (OHPC) which has been used in clinical and veterinary medicine. It has reportedly also been used in birth control pills.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

Acetomepregenol (ACM), also known as mepregenol diacetate and sold under the brand name Diamol, is a progestin medication which is used in Russia for the treatment of gynecological conditions and as a method of birth control in combination with an estrogen. It has also been studied in the treatment of threatened abortion. It has been used in veterinary medicine as well. It has been marketed since at least 1981.

Butagest, also known as buterol, as well as 3β-hydroxy-6-methyl-17α-hydroxypregna-4,6-dien-20-one 3β-butanoate 17α-acetate or as 6-methyl-17α-hydroxy-δ6-progesterone 3β-butanoate 17α-acetate, is a steroidal progestin which was developed in Russia for potential clinical use but was never marketed. It is a modification of megestrol acetate in which the C3 ketone has been replaced with a C3β butanoyloxy moiety.
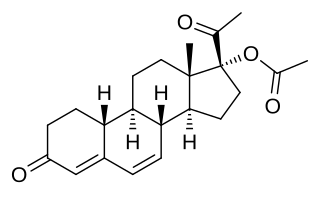
Gestadienol acetate an orally active progestin which was described in the literature in 1967 and was never marketed. It has no androgenic or estrogenic effects. The effects of gestadienol acetate on the endometrium and its general pharmacology were studied in a clinical trial in women. It has also been studied in a clinical trial for benign prostatic hyperplasia in men, but was ineffective.

Cymegesolate, also known as cypionyl megestrol acetate or as megestrol acetate 3β-cypionate, is a progestin medication which was never marketed. It was developed in China in the late 1970s and early to mid 1980s for use as a hormonal contraceptive. The medication was formulated at a dose of 50–100 mg in combination with a "trace" dose of 0.25–0.5 mg quinestrol as a long-lasting, once-a-month combined oral contraceptive pill. This combination has been studied in 1,213 women across a total of 9,651 menstrual cycles, with contraceptive effectiveness of over 99.13% and "very few side effects." At the high dose, it showed an anovulation rate of only about 60%, and instead mediated its contraceptive effects via a marked anti-implantation effect.

Megestrol caproate, abbreviated as MGC, is a progestin medication which was never marketed. It was developed in Russia in 2002. In animals, MGC shows 10-fold higher progestogenic activity compared to progesterone when both are administered via subcutaneous injection. In addition, MGC has no androgenic, anabolic, or estrogenic activity. The medication was suggested as a potential contraceptive and therapeutic agent.

Methenmadinone, also known as deacetylsuperlutin or as 16-methylene-6-dehydro-17α-hydroxyprogesterone, is a pregnane steroid which was never marketed. It is a parent compound of methenmadinone acetate, melengestrol, and chlormethenmadinone.

16-Methylene-17α-hydroxyprogesterone acetate is a progestin of the 17α-hydroxyprogesterone group which was never marketed. Given orally, it shows about 2.5-fold the progestogenic activity of parenteral progesterone in animal bioassays. It is a parent compound of the following clinically used progestins:

Bromethenmadinone acetate is a progestin medication which was developed in Czechoslovakia and was described in 1970 but was never marketed. Analogues of BMMA include chlormethenmadinone acetate, melengestrol acetate, and methenmadinone acetate.


















