| |
 | |
| Clinical data | |
|---|---|
| Other names | Trienolone; Trienbolone; RU-2341; Δ9,11-Nandrolone; 19-Nor-δ9,11-testosterone; Estra-4,9,11-trien-17β-ol-3-one |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intramuscular (as esters) |
| Drug class | Androgen; Anabolic steroid; Progestogen |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | IM : 80-100%[ citation needed ] |
| Metabolism | Liver |
| Elimination half-life | 6–8 hours[ citation needed ] |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.127.177 |
| Chemical and physical data | |
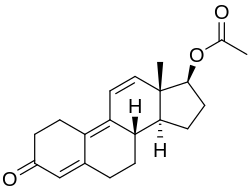
| Formula | C18H22O2 |
| Molar mass | 270.372 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Trenbolone is an androgen and anabolic steroid (AAS) of the nandrolone group which itself was never marketed.[ clarification needed ] [2] [3] [4] [5] [6] Trenbolone ester prodrugs, including trenbolone acetate (brand names Finajet, Finaplix, others) and trenbolone hexahydrobenzylcarbonate (brand names Parabolan, Hexabolan), are or have been marketed for veterinary and clinical use. [2] [3] [4] [6] [7] [8] Trenbolone acetate is used in veterinary medicine in livestock to increase muscle growth and appetite, while trenbolone hexahydrobenzylcarbonate was formerly used clinically in humans but is now no longer marketed. [2] [3] [4] [6] In addition, although it is not approved for clinical or veterinary use, trenbolone enanthate is sometimes sold on the black market under the name trenabol.
Contents
- Uses
- Veterinary
- Side effects
- Pharmacology
- Pharmacodynamics
- Pharmacokinetics
- Chemistry
- History
- Society and culture
- Generic names
- Legal status
- Doping in sports
- Environmental persistence
- See also
- References
- Further reading