
Selective androgen receptor modulators (SARMs) are a class of drugs that selectively activate the androgen receptor in specific tissues, promoting muscle and bone growth while having less effect on male reproductive tissues like the prostate gland.

BMS-564,929 is an investigational selective androgen receptor modulator (SARM) which is being developed by Bristol-Myers Squibb for treatment of the symptoms of age-related decline in androgen levels in men ("andropause"). These symptoms may include depression, loss of muscle mass and strength, reduction in libido and osteoporosis. Treatment with exogenous testosterone is effective in counteracting these symptoms but is associated with a range of side effects, the most serious of which is enlargement of the prostate gland, which can lead to benign prostatic hypertrophy and even prostate cancer. This means there is a clinical need for selective androgen receptor modulators, which produce anabolic effects in some tissues such as muscle and bone, but without stimulating androgen receptors in the prostate.

S-40503 is an investigational selective androgen receptor modulator (SARM) developed by the Japanese company Kaken Pharmaceuticals, which was developed for the treatment of osteoporosis. SARMs are a new class of drugs which produce tissue-specific anabolic effects in some tissues such as muscle and bone, but without stimulating androgen receptors in other tissues such as in the prostate gland, thus avoiding side effects such as benign prostatic hypertrophy which can occur following treatment with unselective androgens like testosterone or anabolic steroids.

LGD-2226 is an investigational selective androgen receptor modulator (SARM), which is being developed for treatment of muscle wasting and osteoporosis.

Enobosarm, also formerly known as ostarine and by the developmental code names GTx-024, MK-2866, and S-22, is a selective androgen receptor modulator (SARM) which is under development for the treatment of androgen receptor-positive breast cancer in women and for improvement of body composition in people taking GLP-1 receptor agonists like semaglutide. It was also under development for a variety of other indications, including treatment of cachexia, Duchenne muscular dystrophy, muscle atrophy or sarcopenia, and stress urinary incontinence, but development for all other uses has been discontinued. Enobosarm was evaluated for the treatment of muscle wasting related to cancer in late-stage clinical trials, and the drug improved lean body mass in these trials, but it was not effective in improving muscle strength. As a result, enobosarm was not approved and development for this use was terminated. Enobosarm is taken by mouth.
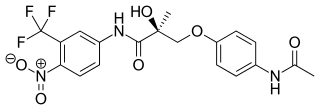
Andarine is a selective androgen receptor modulator (SARM) which was developed by GTX, Inc for the treatment of conditions such as muscle wasting, osteoporosis, and benign prostatic hypertrophy (BPH), using the nonsteroidal antiandrogen bicalutamide as a lead compound. Development of andarine for all indications has been discontinued, in favor of the structurally related and improved compound enobosarm.

Bolandione, also known as 19-norandrostenedione, as well as 19-norandrost-4-en-3,17-dione or estr-4-ene-3,17-dione, is a precursor of the anabolic-androgenic steroid (AAS) nandrolone (19-nortestosterone). Until 2005, bolandione was available without prescription in United States, where it was marketed as a prohormone, but it is now classified as a Schedule III drug. It is also banned from use in many sports, including the Olympic Games, under the World Anti-Doping Code. Bolandione is readily metabolized to nandrolone after oral administration, but its potency to transactivate the androgen receptor dependent reporter gene expression is 10 times lower as compared to dihydrotestosterone (DHT).
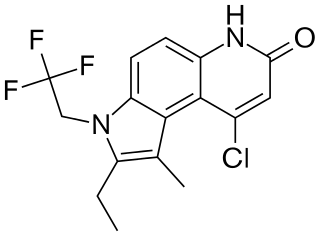
LGD-3303 is a drug which acts as a selective androgen receptor modulator (SARM), with good oral bioavailability. It is a selective agonist for the androgen receptor, producing functional selectivity with effective dissociation of anabolic and androgenic effects, acting as a partial agonist for androgenic effects, but a full agonist for anabolic effects. It has been investigated as a possible treatment for osteoporosis, and was shown in animal studies to enhance the effectiveness of a bisphosphonate drug.
The first antiandrogen was discovered in the 1960s. Antiandrogens antagonise the androgen receptor (AR) and thereby block the biological effects of testosterone and dihydrotestosterone (DHT). Antiandrogens are important for men with hormonally responsive diseases like prostate cancer, benign prostatic hyperplasia (BHP), acne, seborrhea, hirsutism and androgen alopecia. Antiandrogens are mainly used for the treatment of prostate diseases. Research from 2010 suggests that ARs could be linked to the disease progression of triple-negative breast cancer and salivary duct carcinoma and that antiandrogens can potentially be used to treat it.

LGD-4033, also known by the developmental code name VK5211 and by the black-market name Ligandrol, is a selective androgen receptor modulator (SARM) which is under development for the treatment of muscle atrophy in people with hip fracture. It was also under development for the treatment of cachexia, hypogonadism, and osteoporosis, but development for these indications was discontinued. LGD-4033 has been reported to dose-dependently improve lean body mass and muscle strength in preliminary clinical trials, but is still being developed and has not been approved for medical use. The drug is taken by mouth.

Vosilasarm, also known by the development codes RAD140 and EP0062 and by the black-market name Testolone or Testalone, is a selective androgen receptor modulator (SARM) which is under development for the treatment of hormone-sensitive breast cancer. It is specifically under development for the treatment of androgen receptor-positive, estrogen receptor-negative, HER2-negative advanced breast cancer. Vosilasarm was also previously under development for the treatment of sarcopenia, osteoporosis, and weight loss due to cancer cachexia, but development for these indications was discontinued. The drug is taken by mouth.

Acetothiolutamide is a selective androgen receptor modulator (SARM) derived from the nonsteroidal antiandrogen bicalutamide that was described in 2002 and was one of the first SARMs to be discovered and developed. It is a high-affinity, selective ligand of the androgen receptor (AR), where it acts as a full agonist in vitro, and has in vitro potency comparable to that of testosterone. However, in vivo, acetothiolutamide displayed overall negligible androgenic effects, though significant anabolic effects were observed at high doses. In addition, notable antiandrogen effects were observed in castrated male rats treated with testosterone propionate. The discrepancy between the in vitro and in vivo actions of acetothiolutamide was determined to be related to rapid plasma clearance and extensive hepatic metabolism into a variety of metabolites with differing pharmacological activity, including AR partial agonism and antagonism. In accordance with its poor metabolic stability, acetothiolutamide is not orally bioavailable, and shows activity only via injected routes such as subcutaneous and intravenous.

LG121071 is a selective androgen receptor modulator (SARM) developed by Ligand Pharmaceuticals that was first described in 1999 and was the first orally active nonsteroidal androgen to be discovered. It is a tricyclic quinolone derivative, structurally distinct from other nonsteroidal AR agonists like andarine and enobosarm (ostarine). The drug acts as a high-affinity full agonist of the androgen receptor (AR), with a potency and efficacy that is said to be equivalent to that of dihydrotestosterone (DHT). Unlike testosterone, but similarly to DHT, LG121071 and other nonsteroidal androgens cannot be potentiated by 5α-reductase in androgenic tissues, and for this reason, show tissue-selective androgenic effects. In accordance, they are said to possess full anabolic activity with reduced androgenic activity, similarly to anabolic-androgenic steroids.

TFM-4AS-1 is a dual selective androgen receptor modulator (SARM) and 5α-reductase inhibitor. It is a potent and selective partial agonist (Emax = 55%) of the androgen receptor (IC50 = 30 nM) and inhibitor of 5α-reductase types I and II (IC50 = 2 and 3 nM, respectively). TFM-4AS-1 shows tissue-selective androgenic effects; it promotes the accumulation of bone and muscle mass and has reduced effects in reproductive tissues and sebaceous glands. In an animal study, TFM-4AS-1 stimulated sebaceous gland formation only 31% as much as dihydrotestosterone (DHT) at doses that were as anabolic or more so than DHT. In addition, TFM-4AS-1 only weakly promoted growth of the prostate gland and it partially antagonized the actions of DHT in the seminal vesicles and endogenous androgens in the prostate gland. Structurally, TFM-4AS-1 is a 4-azasteroid. A structurally related and more advanced version of TFM-4AS-1, MK-0773, was developed and pursued for potential pharmaceutical use.

The pharmacology of bicalutamide is the study of the pharmacodynamic and pharmacokinetic properties of the nonsteroidal antiandrogen (NSAA) bicalutamide. In terms of pharmacodynamics, bicalutamide acts as a selective antagonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). It has no capacity to activate the AR. It does not decrease androgen levels and has no other important hormonal activity. The medication has progonadotropic effects due to its AR antagonist activity and can increase androgen, estrogen, and neurosteroid production and levels. This results in a variety of differences of bicalutamide monotherapy compared to surgical and medical castration, such as indirect estrogenic effects and associated benefits like preservation of sexual function and drawbacks like gynecomastia. Bicalutamide can paradoxically stimulate late-stage prostate cancer due to accumulated mutations in the cancer. When used as a monotherapy, bicalutamide can induce breast development in males due to its estrogenic effects. Unlike other kinds of antiandrogens, it may have less adverse effect on the testes and fertility.

JNJ-28330835 is a drug which acts as a selective androgen receptor modulator (SARM). In studies on rats it was found to enhance muscle growth and sexual behavior but with minimal effects on prostate gland size. A number of related compounds are known, though JNJ-28330835 has progressed furthest through development.
Arcarine (ORM-11984) is a selective androgen receptor modulator (SARM) developed by Orion Corporation, a Finnish pharmaceutical company. It belongs to a class of drugs designed to have tissue-selective androgenic effects, potentially offering the benefits of androgens while minimizing unwanted side effects. Arcarine was investigated for the treatment of various conditions, including benign prostatic hyperplasia, hypogonadism, and osteoporosis. The compound reached Phase I clinical trials before development was discontinued. Like other SARMs, Arcarine was developed to potentially provide anabolic effects in muscle and bone tissue while having reduced androgenic effects in other tissues, such as the prostate.

JNJ-26146900 is a selective androgen receptor modulator (SARM) which was developed by Johnson & Johnson for the potential treatment of prostate cancer but was never marketed.

GTx-027 is a selective androgen receptor modulator (SARM) which was under development for or of potential interest in the treatment of breast cancer and stress urinary incontinence (SUI) but was never marketed. It is taken by mouth.

MK-4541 is a dual selective androgen receptor modulator (SARM) and 5α-reductase inhibitor (5α-RI) which has been of interest for the potential treatment of prostate cancer but has not been marketed at this time. It is intended for use by mouth.

















