Antihypertensives are a class of drugs that are used to treat hypertension. Antihypertensive therapy seeks to prevent the complications of high blood pressure, such as stroke, heart failure, kidney failure and myocardial infarction. Evidence suggests that reduction of the blood pressure by 5 mmHg can decrease the risk of stroke by 34% and of ischaemic heart disease by 21%, and can reduce the likelihood of dementia, heart failure, and mortality from cardiovascular disease. There are many classes of antihypertensives, which lower blood pressure by different means. Among the most important and most widely used medications are thiazide diuretics, calcium channel blockers, ACE inhibitors, angiotensin II receptor antagonists (ARBs), and beta blockers.
Spironolactone, sold under the brand name Aldactone among others, is a diuretic medication primarily used to treat fluid build-up due to heart failure, liver scarring, or kidney disease. It is also used in the treatment of high blood pressure, and low blood potassium that does not improve with supplementation, early puberty in boys, acne and excessive hair growth in women. Spironolactone is taken by mouth.

Amiloride, sold under the trade name Midamor among others, is a medication typically used with other medications to treat high blood pressure or swelling due to heart failure or cirrhosis of the liver. Amiloride is classified as a potassium-sparing diuretic. Amiloride is often used together with another diuretic, such as a thiazide or loop diuretic. It is taken by mouth. Onset of action is about two hours and it lasts for about a day.

Potassium-sparing diuretics or antikaliuretics refer to drugs that cause diuresis without causing potassium loss in the urine. They are typically used as an adjunct in management of hypertension, cirrhosis, and congestive heart failure. The steroidal aldosterone antagonists can also be used for treatment of primary hyperaldosteronism. Spironolactone, a steroidal aldosterone antagonist, is also used in management of female hirsutism and acne from PCOS or other causes.

Drospirenone is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy and in menopausal hormone therapy, among other uses. It is available both alone under the brand name Slynd and in combination with an estrogen under the brand name Yasmin among others. The medication is an analog of the drug spironolactone. Drospirenone is taken by mouth.

A mineralocorticoid receptor antagonist or aldosterone antagonist, is a diuretic drug which antagonizes the action of aldosterone at mineralocorticoid receptors. This group of drugs is often used as adjunctive therapy, in combination with other drugs, for the management of chronic heart failure. Spironolactone, the first member of the class, is also used in the management of hyperaldosteronism and female hirsutism. Most antimineralocorticoids, including spironolactone, are steroidal spirolactones. Finerenone is a nonsteroidal antimineralocorticoid.

Eplerenone, sold under the brand name Inspra, is an aldosterone antagonist type of potassium-sparing diuretic that is used to treat chronic heart failure and high blood pressure, particularly for people with resistant hypertension due to elevated aldosterone. It is a steroidal antimineralocorticoid of the spirolactone group and a selective aldosterone receptor antagonist (SARA).

Potassium canrenoate or canrenoate potassium (USAN), also known as aldadiene kalium, the potassium salt of canrenoic acid, is an aldosterone antagonist of the spirolactone group. Like spironolactone, it is a prodrug, and is metabolized to active canrenone in the body.

Spirolactones are a class of functional group in organic chemistry featuring a cyclic ester attached spiro to another ring system. The name is also used to refer to a class of synthetic steroids, called steroid-17α-spirolactones, 17α-spirolactosteroids, or simply 17α-spirolactones, which feature their spirolactone group at the C17α position. They are antimineralocorticoids, or antagonists of the mineralocorticoid receptor, and have been employed clinically as potassium-sparing diuretics. Some also possess progestogenic and/or antiandrogen properties, which have both contributed to side effects and been utilized for medical indications. The spirolactones were developed by G. D. Searle & Company in the 1950s and thereafter and were denoted as "SC" compounds.

Prorenone is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed. It is the lactonic form of prorenoic acid (prorenoate), and prorenoate potassium (SC-23992), the potassium salt of prorenoic acid, also exists. Prorenoate potassium is about 8 times more potent than spironolactone as an antimineralocorticoid in animals, and it may act as a prodrug to prorenone. In addition to the mineralocorticoid receptor, prorenone also binds to the glucocorticoid, androgen, and progesterone receptors. The antiandrogenic potency of prorenone in vivo in animals is close to that of spironolactone. Similarly to spironolactone, prorenone is also a potent inhibitor of aldosterone biosynthesis.

Mexrenone is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed. It is the lactonic form of mexrenoic acid (mexrenoate), and mexrenoate potassium (SC-26714), the potassium salt of mexrenoic acid, also exists. In addition to the mineralocorticoid receptor, mexrenone also binds to the glucocorticoid, androgen, and progesterone receptors. Relative to spironolactone, it has markedly reduced antiandrogen activity. Eplerenone is the 9-11α-epoxy analogue of mexrenone.

SC-5233, also known as 6,7-dihydrocanrenone or 20-spirox-4-ene-3,20-dione, is a synthetic, steroidal antimineralocorticoid of the spirolactone group which was developed by G. D. Searle & Company in the 1950s but was never marketed. It was the first synthetic antagonist of the mineralocorticoid receptor to have been identified and tested in humans. The drug was found to lack appreciable oral bioavailability and to be of low potency when administered parenterally, but it nonetheless produced a mild diuretic effect in patients with congestive heart failure. SC-8109, the 19-nor (19-demethyl) analogue, was developed and found to have improved oral bioavailability and potency, but still had low potency. Spironolactone followed and had both good oral bioavailability and potency, and was the first synthetic antimineralocorticoid to be marketed. It has about 46-fold higher oral potency than SC-5233.

Mespirenone (INN), also known as Δ1-15β,16β-methylenespironolactone, is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed. Animal research found that it was 3.3-fold more potent as an antimineralocorticoid relative to spironolactone. In addition to its antimineralocorticoid properties, mespirenone is also a progestogen, antigonadotropin, and antiandrogen. It is 2- to 3-fold as potent as spironolactone as a progestogen and antigonadotropin but its antiandrogenic activity is markedly reduced and weak in comparison. Mespirenone is also a potent and specific enzyme inhibitor of 18-hydroxylase and thus of mineralocorticoid biosynthesis. The drug was under development by Schering and reached phase II clinical trials but was discontinued in 1989.

SC-8109 is a steroidal antimineralocorticoid of the spirolactone group which was never marketed. It is a potent antagonist of the mineralocorticoid receptor and is more potent than the related drug SC-5233. However, SC-8109 was found to have relatively low oral bioavailability and potency, though it nonetheless produced a mild diuretic effect in patients with congestive heart failure. Spironolactone, another spirolactone, followed and had both good oral bioavailability and potency, and was the first antimineralocorticoid to be marketed.

←

6β-Hydroxy-7α-thiomethylspironolactone (6β-OH-7α-TMS) is a steroidal antimineralocorticoid of the spirolactone group and a major active metabolite of spironolactone. Other important metabolites of spironolactone include 7α-thiospironolactone, 7α-thiomethylspironolactone, and canrenone (SC-9376).

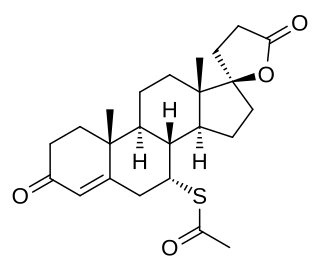
7α-Thiospironolactone is a steroidal antimineralocorticoid and antiandrogen of the spirolactone group and a minor active metabolite of spironolactone. Other important metabolites of spironolactone include 7α-thiomethylspironolactone, 6β-hydroxy-7α-thiomethylspironolactone (6β-OH-7α-TMS), and canrenone (SC-9376).

7α-Thioprogesterone is a synthetic, steroidal, and potent antimineralocorticoid (putative) and antiandrogen which was developed by G. D. Searle & Co and was described in the late 1970s and early 1980s but was never developed or introduced for medical use. It is a derivative of progesterone (pregn-4-ene-3,20-dione) with a thio (sulfur) substitution at the C7α position, and is related to the spirolactone group of drugs but lacks a γ-lactone ring.

The pharmacodynamics of spironolactone, an antimineralocorticoid and antiandrogen medication, concern its mechanisms of action, including its biological targets and activities, as well as its physiological effects. The pharmacodynamics of spironolactone are characterized by high antimineralocorticoid activity, moderate antiandrogenic activity, and weak steroidogenesis inhibition. In addition, spironolactone has sometimes been found to increase estradiol and cortisol levels and hence could have slight indirect estrogenic and glucocorticoid effects. The medication has also been found to interact very weakly with the estrogen and progesterone receptors, and to act as an agonist of the pregnane X receptor. Likely due to increased activation of the estrogen and/or progesterone receptors, spironolactone has very weak but significant antigonadotropic effects.
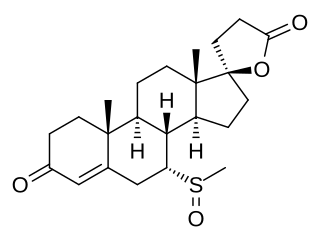
7α-Thiomethylspironolactone sulfoxide is a metabolite of spironolactone, an antimineralocorticoid and antiandrogen medication. 7α-TMS sulfoxide is specifically formed from 7α-thiomethylspironolactone (7α-TMS).


















