
Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.

Gestonorone caproate, also known as gestronol hexanoate or norhydroxyprogesterone caproate and sold under the brand names Depostat and Primostat, is a progestin medication which is used in the treatment of enlarged prostate and cancer of the endometrium. It is given by injection into muscle typically once a week.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.
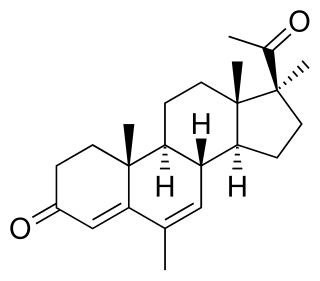
Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. It is taken by mouth.

Promegestone, sold under the brand name Surgestone, is a progestin medication which is used in menopausal hormone therapy and in the treatment of gynecological disorders. It is taken by mouth.

Demegestone, sold under the brand name Lutionex, is a progestin medication which was previously used to treat luteal insufficiency but is now no longer marketed. It is taken by mouth.

Trimegestone, sold under the brand names Ondeva and Totelle among others, is a progestin medication which is used in menopausal hormone therapy and in the prevention of postmenopausal osteoporosis. It was also under development for use in birth control pills to prevent pregnancy, but ultimately was not marketed for this purpose. The medication is available alone or in combination with an estrogen. It is taken by mouth.

Nomegestrol acetate (NOMAC), sold under the brand names Lutenyl and Zoely among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. NOMAC is taken by mouth. A birth control implant for placement under the skin was also developed but ultimately was not marketed.

Segesterone acetate (SGA), sold under the brand names Nestorone, Elcometrine, and Annovera, is a progestin medication which is used in birth control and in the treatment of endometriosis in the United States, Brazil, and other South American countries. It is available both alone and in combination with an estrogen. It is not effective by mouth and must be given by other routes, most typically as a vaginal ring or implant that is placed into fat.
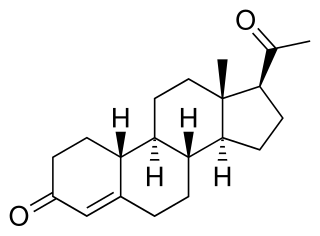
19-Norprogesterone, also known as 19-norpregn-4-ene-3,20-dione, is a steroidal progestin and close analogue of the sex hormone progesterone, lacking only the C19 methyl group of that molecule. It was first synthesized in 1944 in the form of a mixture that also included unnatural stereoisomers of progesterone, and this mixture was found to be at least equivalent to progesterone in terms of progestogenic activity. Subsequent investigations revealed that 17-isoprogesterone and 14-iso-17-isoprogesterone are devoid of progestogenic activity. 19-Norprogesterone was resynthesized in 1951 with an improved method, and was confirmed to be the component of the mixture synthesized in 1944 that was responsible for its progestogenic activity. In 1953, a paper was published showing that 19-norprogesterone possessed 4- to 8-fold the activity of progesterone in the Clauberg assay in rabbits, and at the time of this discovery, 19-norprogesterone was the most potent progestogen known.

Norvinisterone, sold under the brand names Neoprogestin and Nor-Progestelea, is a progestin and androgen/anabolic steroid (AAS) medication which was used in Europe but is now no longer marketed. It is taken by mouth.

3β-Dihydroprogesterone (3β-DHP), also known as 3β-hydroxyprogesterone, or pregn-4-en-3β-ol-20-one, is an endogenous steroid. It is biosynthesized by 3β-hydroxysteroid dehydrogenase from progesterone. Unlike 3α-dihydroprogesterone (3α-DHP), 3β-DHP does not act as a positive allosteric modulator of the GABAA receptor, which is in accordance with the fact that other 3β-hydroxylated progesterone metabolites such as isopregnanolone and epipregnanolone similarly do not act as potentiators of this receptor and instead inhibit it as well as reverse the effects of potentiators like allopregnanolone. 3β-DHP has been reported to possess about the same potency as progesterone in a bioassay of progestogenic activity, whereas 3α-DHP was not assessed.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously used in birth control pills in Italy but is now no longer marketed. It is taken by mouth.

17α-Methylprogesterone (17α-MP), or 17α-methylpregn-4-ene-3,20-dione, is a steroidal progestin related to progesterone that was synthesized and characterized in 1949 but was never marketed. Along with ethisterone (1938) and 19-norprogesterone (1951), 17α-MP was one of the earliest derivatives of progesterone to be identified as possessing progestogenic activity. Similarly to progesterone and derivatives like 17α-hydroxyprogesterone and 19-norprogesterone, 17α-MP was found to possess poor oral bioavailability, but showed improved progestogenic activity relative to progesterone when administered via other routes. In addition to its activity as a progestogen, 17α-MP has also been found to possess some antiglucocorticoid activity.

Progesterone 3-acetyl enol ether, also known as progesterone acetate, as well as 3-acetoxypregna-3,5-dien-20-one, is a progestin which was never marketed. It was reported to possess similar potency to progesterone and hydroxyprogesterone caproate in the rabbit endometrial carbonic anhydrase test, a bioassay of progestogenic activity. In addition, it was able to maintain pregnancy in animals. Progesterone 3-acetyl enol ether is closely related to quingestrone, which is also known as progesterone 3-cyclopentyl enol ether and was formerly marketed as an oral contraceptive.
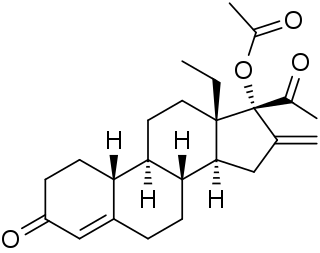
18-Methylsegesterone acetate is a progestin medication of the 19-norprogesterone group which was never marketed. It was first described in a patent in 1997 and then in a literature paper in 2003. 18-Methyl-SGA is the C18 methyl or C13β ethyl derivative of segesterone acetate, and shows 3 to 10 times the progestogenic potency of SGA in bioassays. This is analogous to the case of the 19-nortestosterone progestin norethisterone and its 18-methyl derivative levonorgestrel, the latter showing substantially increased potency relative to the former similarly. As SGA is already one of the most potent progestins to have been developed, with 100-fold the potency of progesterone and 10-fold the potency of levonorgestrel in bioassays, 18-methyl-SGA is an extremely potent progestogen, among if not the most potent known.

Ethinylandrostenediol, also known as 17α-ethynyl-5-androstenediol, is a synthetic estrogen, progestogen, and androgen which was never marketed. It is the C17α ethynyl derivative of the androgen precursor and prohormone 5-androstenediol.



















