Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.

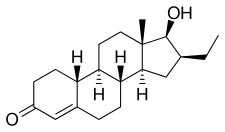
Bicalutamide, sold under the brand name Casodex among others, is an antiandrogen medication that is primarily used to treat prostate cancer. It is typically used together with a gonadotropin-releasing hormone (GnRH) analogue or surgical removal of the testicles to treat metastatic prostate cancer (mPC). To a lesser extent, it is used at high doses for locally advanced prostate cancer (LAPC) as a monotherapy without castration. Bicalutamide was also previously used as monotherapy to treat localized prostate cancer (LPC), but authorization for this use was withdrawn following unfavorable trial findings. Besides prostate cancer, bicalutamide is limitedly used in the treatment of excessive hair growth and scalp hair loss in women, as a puberty blocker and component of feminizing hormone therapy for transgender girls and women, to treat gonadotropin-independent early puberty in boys, and to prevent overly long-lasting erections in men. It is taken by mouth.

Gestonorone caproate, also known as gestronol hexanoate or norhydroxyprogesterone caproate and sold under the brand names Depostat and Primostat, is a progestin medication which is used in the treatment of enlarged prostate and cancer of the endometrium. It is given by injection into muscle typically once a week.

Trestolone, also known as 7α-methyl-19-nortestosterone (MENT), is an experimental androgen/anabolic steroid (AAS) and progestogen medication which has been under development for potential use as a form of hormonal birth control for men and in androgen replacement therapy for low testosterone levels in men but has never been marketed for medical use. It is given as an implant that is placed into fat. As trestolone acetate, an androgen ester and prodrug of trestolone, the medication can also be given by injection into muscle.

Hydroxyprogesterone caproate, sold under the brand name Delalutin among others, is a medication used to reduce the risk of preterm birth in women pregnant with one baby who have a history of spontaneous preterm birth. In March 2023, the manufacturer, Covis Pharma, agreed to withdraw the drug from the US market. The approval of this drug substance was withdrawn by the US Food and Drug Administration (FDA) in April 2023. In May 2024, the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency recommended suspending the marketing authorizations of medications containing 17-hydroxyprogesterone caproate in the European Union.
Feminizing hormone therapy, also known as transfeminine hormone therapy, is a form of gender-affirming care and a gender-affirming hormone therapy to change the secondary sex characteristics of transgender people from masculine to feminine. It is a common type of transgender hormone therapy and is used to treat transgender women and non-binary transfeminine individuals. Some, in particular intersex people, but also some non-transgender people, take this form of therapy according to their personal needs and preferences.
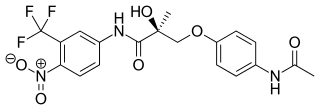
Andarine is a selective androgen receptor modulator (SARM) which was developed by GTX, Inc for the treatment of conditions such as muscle wasting, osteoporosis, and benign prostatic hyperplasia (BPH), using the nonsteroidal antiandrogen bicalutamide as a lead compound. Development of andarine for all indications has been discontinued, in favor of the structurally related and improved compound enobosarm.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Allylestrenol, sold under the brand names Gestanin and Turinal among others, is a progestin medication which is used to treat recurrent and threatened miscarriage and to prevent premature labor in pregnant women. However, except in the case of proven progesterone deficiency, its use for such purposes is no longer recommended. It is also used in Japan to treat benign prostatic hyperplasia (BPH) in men. The medication is used alone and is not formulated in combination with an estrogen. It is taken by mouth.

Estradiol undecylate, also known as estradiol undecanoate and formerly sold under the brand names Delestrec and Progynon Depot 100 among others, is an estrogen medication which has been used in the treatment of prostate cancer in men. It has also been used as a part of hormone therapy for transgender women. Although estradiol undecylate has been used in the past, it was discontinued. The medication has been given by injection into muscle usually once a month.

Cyproterone acetate (CPA), sold alone under the brand name Androcur or with ethinylestradiol under the brand names Diane or Diane-35 among others, is an antiandrogen and progestin medication used in the treatment of androgen-dependent conditions such as acne, excessive body hair growth, early puberty, and prostate cancer, as a component of feminizing hormone therapy for transgender individuals, and in birth control pills. It is formulated and used both alone and in combination with an estrogen. CPA is taken by mouth one to three times per day.

Cyproterone, also known by its developmental code name SH-80881, is a steroidal antiandrogen which was studied in the 1960s and 1970s but was never introduced for medical use. It is a precursor of cyproterone acetate (CPA), an antiandrogen, progestin, and antigonadotropin which was introduced instead of cyproterone and is widely used as a medication. Cyproterone and CPA were among the first antiandrogens to be developed.

Osaterone acetate, sold under the brand name Ypozane, is a medication which is used in veterinary medicine for the treatment of enlarged prostate in dogs. It is given by mouth.
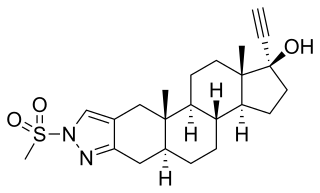
Zanoterone, also known as (5α,17α)-1'-(methylsulfonyl)-1'-H-pregn-20-yno[3,2-c]pyrazol-17-ol, is a steroidal antiandrogen which was never marketed. It was investigated for the treatment of benign prostatic hyperplasia (BPH) but failed to demonstrate sufficient efficacy in phase II clinical trials, and also showed an unacceptable incidence rate and severity of side effects. As such, it was not further developed.

Bifluranol is a synthetic nonsteroidal estrogen of the stilbestrol group related to diethylstilbestrol that has been used as an antiandrogen in the United Kingdom in the treatment of benign prostatic hyperplasia. The drug is described as a weak estrogen, and possesses about one-eighth the potency of diethylstilbestrol.

BOMT, also known by its developmental code name Ro 7-2340 and as 6α-bromo-4-oxa-17α-methyl-5α-dihydrotestosterone, is a synthetic steroidal antiandrogen which was first produced in 1970 and was never marketed for medical use. It is the 6α-brominated, 4-oxygenated, and 17α-methylated derivative of the androgen dihydrotestosterone (DHT). Along with benorterone, cyproterone, and flutamide, BOMT was among the earliest antiandrogens to be developed and extensively studied, although it is less well-documented in comparison to the others. BOMT has been investigated clinically in the treatment of benign prostatic hyperplasia, though development for this use did not continue. There was also interest in BOMT for the potential applications of acne, pattern hair loss, and possibly prostate cancer, but it was not developed for these indications either.

Trimethyltrienolone (TMT), also known by its developmental code name R-2956 or RU-2956, is an antiandrogen medication which was never introduced for medical use but has been used in scientific research.

A steroidal antiandrogen (SAA) is an antiandrogen with a steroidal chemical structure. They are typically antagonists of the androgen receptor (AR) and act both by blocking the effects of androgens like testosterone and dihydrotestosterone (DHT) and by suppressing gonadal androgen production. SAAs lower concentrations of testosterone through simulation of the negative feedback inhibition of the hypothalamus. SAAs are used in the treatment of androgen-dependent conditions in men and women, and are also used in veterinary medicine for the same purpose. They are the converse of nonsteroidal antiandrogens (NSAAs), which are antiandrogens that are not steroids and are structurally unrelated to testosterone.
The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.

The pharmacology of cyproterone acetate (CPA) concerns the pharmacology of the steroidal antiandrogen and progestin medication cyproterone acetate.