
A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.
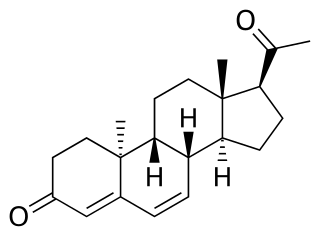
Dydrogesterone, sold under the brand name Duphaston among others, is a progestin medication which is used for a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy. It is taken by mouth.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.

Demegestone, sold under the brand name Lutionex, is a progestin medication which was previously used to treat luteal insufficiency but is now no longer marketed. It is taken by mouth.

Cyproterone, also known by its developmental code name SH-80881, is a steroidal antiandrogen which was studied in the 1960s and 1970s but was never introduced for medical use. It is an analogue of cyproterone acetate (CPA), an antiandrogen, progestin, and antigonadotropin which was introduced instead of cyproterone and is widely used as a medication. Cyproterone and CPA were among the first antiandrogens to be developed.

Delmadinone acetate (DMA), sold under the brand name Tardak among others, is a progestin and antiandrogen which is used in veterinary medicine to treat androgen-dependent conditions such as benign prostatic hyperplasia. It must be used with care as it has the potential to cause adrenal insufficiency via inhibition of adrenocorticotropic hormone (ACTH) secretion from the pituitary gland. DMA is the C17α acetate ester of delmadinone, which, in contrast to DMA, was never marketed for medical use.

Segesterone acetate (SGA), sold under the brand names Nestorone, Elcometrine, and Annovera, is a progestin medication which is used in birth control and in the treatment of endometriosis in the United States, Brazil, and other South American countries. It is available both alone and in combination with an estrogen. It is not effective by mouth and must be given by other routes, most typically as a vaginal ring or implant that is placed into fat.

Osaterone acetate, sold under the brand name Ypozane, is a medication which is used in veterinary medicine in Europe in the treatment of enlarged prostate in dogs. It is given by mouth.

Retroprogesterone, also known as 9β,10α-progesterone or as 9β,10α-pregn-4-ene-3,20-dione, is a progestin which was never marketed. It is a stereoisomer of the naturally occurring progestogen progesterone, in which the hydrogen atom at the 9th carbon is in the α-position instead of the β-position and the methyl group at the 10th carbon is in the β-position instead of the α-position. In other words, the atom positions at the two carbons have been reversed relative to progesterone, hence the name retroprogesterone. This reversal results in a "bent" configuration in which the plane of rings A and B is orientated at a 60° angle below the rings C and D. This configuration is ideal for interaction with the progesterone receptor, with retroprogesterone binding with high affinity to this receptor. However, the configuration is not as ideal for binding to other steroid hormone receptors, and as a result, retroprogesterone derivatives have increased selectivity for the progesterone receptor relative to progesterone.

Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously used in birth control pills in Italy but is now no longer marketed. It is taken by mouth.

Dimethandrolone (DMA), also known by its developmental code name CDB-1321, is an experimental androgen/anabolic steroid (AAS) and progestogen medication which is under investigation for potential clinical use.

Flumedroxone acetate, sold under the brand names Demigran and Leomigran, is a progestin medication which is or has been used as an antimigraine agent. It is taken by mouth.

Ro 6-3129, also known as 16α-ethylthio-6-dehydroretroprogesterone or as 16α-ethylthio-9β,10α-pregna-4,6-diene-3,20-dione, as well as 16α-ethylthiodydrogesterone, is a progestogen of the retroprogesterone group which was developed by Roche but was never marketed. It shows greater potency than dydrogesterone in bioassays.
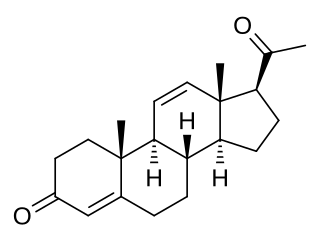
11-Dehydroprogesterone, also known as pregna-4,11-diene-3,20-dione, is a steroidal progestin that was never marketed. It was found to be 2- to 3-fold as potent as progesterone as a progestogen in animal bioassays, although other studies found them to be equivalent in potency. 11-Dehydroprogesterone has been studied in women. It was discovered in the 1930s or 1940s, and was one of the earliest synthetic progestogens.
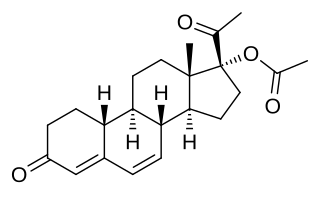
Gestadienol acetate an orally active progestin which was described in the literature in 1967 and was never marketed. It has no androgenic or estrogenic effects. The effects of gestadienol acetate on the endometrium and its general pharmacology were studied in a clinical trial in women. It has also been studied in a clinical trial for benign prostatic hyperplasia in men, but was ineffective.

20α-Dihydrotrengestone (20α-DHTG), also known as 20α-hydroxytrengestone, as well as 6-chloro-20(S)-hydroxy-9β,10α-pregna-1,4,6-trien-3-one, is a progestin and the major active metabolite of trengestone. It appears that trengestone is a prodrug of 20α-DHTG, as it is largely transformed into this metabolite when given orally in humans. 20α-DHTG has potent progestogenic activity similarly to trengestone.

DU-41164, also known as 1,2β-methylene-6-fluoro-17α-acetoxy-δ6-retroprogesterone, is a progestin which was developed by Philips-Duphar in the 1970s and was never marketed. It is a combined derivative of 17α-hydroxyprogesterone and retroprogesterone. The drug shows extremely high potency as a progestogen in animals; it was reported to possess 500 times the affinity of progesterone for the progesterone receptor expressed in rabbit uterus, and showed 600 times the progestogenic potency of subcutaneous progesterone when given orally in animals. The affinity of DU-41164 for the progesterone receptor was described in 1974 as "probably the highest reported for any steroid-receptor interaction". The drug showed no androgenic, anabolic, antiandrogenic, estrogenic, or corticosteroid activity in animals. Although highly potent in animals, DU-41164 produced little or no progestogenic effect at dosages of 50 and 200 µg/day in women, suggesting major species differences. A closely related compound, DU-41165, has been developed as a photoaffinity label for the progesterone receptor.

Ethinylandrostenediol, also known as 17α-ethynyl-5-androstenediol, is a synthetic estrogen, progestogen, and androgen which was never marketed. It is the C17α ethynyl derivative of the androgen precursor and prohormone 5-androstenediol.




















