Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.
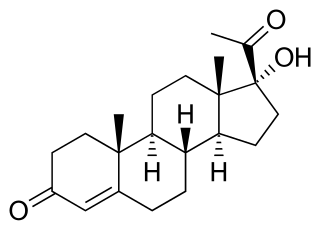
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.

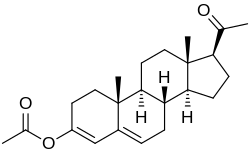
Gestonorone caproate, also known as gestronol hexanoate or norhydroxyprogesterone caproate and sold under the brand names Depostat and Primostat, is a progestin medication which is used in the treatment of enlarged prostate and cancer of the endometrium. It is given by injection into muscle typically once a week.

Hydroxyprogesterone caproate (OHPC), sold under the brand names Proluton and Makena among others, is a progestin medication which is used to prevent preterm birth in pregnant women with a history of the condition and to treat gynecological disorders. It has also been formulated in combination with estrogens for various indications and as a form of long-lasting injectable birth control. It is not used by mouth and is instead given by injection into muscle or fat, typically once per week to once per month depending on the indication.
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.

Hydroxyprogesterone acetate (OHPA), sold under the brand name Prodox, is an orally active progestin related to hydroxyprogesterone caproate (OHPC) which has been used in clinical and veterinary medicine. It has reportedly also been used in birth control pills.

3β-Dihydroprogesterone (3β-DHP), also known as 3β-hydroxyprogesterone, or pregn-4-en-3β-ol-20-one, is an endogenous steroid. It is biosynthesized by 3β-hydroxysteroid dehydrogenase from progesterone. Unlike 3α-dihydroprogesterone (3α-DHP), 3β-DHP does not act as a positive allosteric modulator of the GABAA receptor, which is in accordance with the fact that other 3β-hydroxylated progesterone metabolites such as isopregnanolone and epipregnanolone similarly do not act as potentiators of this receptor and instead inhibit it as well as reverse the effects of potentiators like allopregnanolone. 3β-DHP has been reported to possess about the same potency as progesterone in a bioassay of progestogenic activity, whereas 3α-DHP was not assessed.

Hydroxyprogesterone heptanoate (OHPH), also known as hydroxyprogesterone enanthate (OHPE) and sold under the brand names H.O.P., Lutogil A.P., and Lutogyl A.P. among others, is a progestin medication used for progestogenic indications. It has been formulated both alone and in together with estrogens, androgens/anabolic steroids, and other progestogens in several combination preparations. OHPH is given by injection into muscle at regular intervals.

Retroprogesterone, also known as 9β,10α-progesterone or as 9β,10α-pregn-4-ene-3,20-dione, is a progestin which was never marketed. It is a stereoisomer of the naturally occurring progestogen progesterone, in which the hydrogen atom at the 9th carbon is in the α-position instead of the β-position and the methyl group at the 10th carbon is in the β-position instead of the α-position. In other words, the atom positions at the two carbons have been reversed relative to progesterone, hence the name retroprogesterone. This reversal results in a "bent" configuration in which the plane of rings A and B is orientated at a 60° angle below the rings C and D. This configuration is ideal for interaction with the progesterone receptor, with retroprogesterone binding with high affinity to this receptor. However, the configuration is not as ideal for binding to other steroid hormone receptors, and as a result, retroprogesterone derivatives have increased selectivity for the progesterone receptor relative to progesterone.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

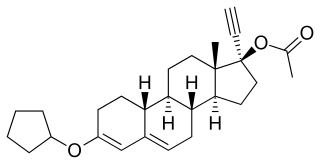
Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously used in birth control pills in Italy but is now no longer marketed. It is taken by mouth.

Pentagestrone acetate (PGA), sold under the brand names Gestovis and Gestovister, is a progestin which was described in the literature in 1960 and was introduced by Vister in Italy in 1961. It is the 3-cyclopentyl enol ether of 17α-hydroxyprogesterone acetate. PGA, along with quingestrone, is said to have very similar properties to those of dydrogesterone, a pure progestogen and close analogue of progesterone.

17α-Methylprogesterone (17α-MP), or 17α-methylpregn-4-ene-3,20-dione, is a steroidal progestin related to progesterone that was synthesized and characterized in 1949 but was never marketed. Along with ethisterone (1938) and 19-norprogesterone (1951), 17α-MP was one of the earliest derivatives of progesterone to be identified as possessing progestogenic activity. Similarly to progesterone and derivatives like 17α-hydroxyprogesterone and 19-norprogesterone, 17α-MP was found to possess poor oral bioavailability, but showed improved progestogenic activity relative to progesterone when administered via other routes. In addition to its activity as a progestogen, 17α-MP has also been found to possess some antiglucocorticoid activity.
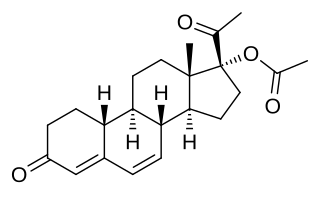
Gestadienol acetate an orally active progestin which was described in the literature in 1967 and was never marketed. It has no androgenic or estrogenic effects. The effects of gestadienol acetate on the endometrium and its general pharmacology were studied in a clinical trial in women. It has also been studied in a clinical trial for benign prostatic hyperplasia in men, but was ineffective.

Cymegesolate, also known as cypionyl megestrol acetate or as megestrol acetate 3β-cypionate, is a progestin medication which was never marketed. It was developed in China in the late 1970s and early to mid 1980s for use as a hormonal contraceptive. The medication was formulated at a dose of 50–100 mg in combination with a "trace" dose of 0.25–0.5 mg quinestrol as a long-lasting, once-a-month combined oral contraceptive pill. This combination has been studied in 1,213 women across a total of 9,651 menstrual cycles, with contraceptive effectiveness of over 99.13% and "very few side effects." At the high dose, it showed an anovulation rate of only about 60%, and instead mediated its contraceptive effects via a marked anti-implantation effect.

Megestrol caproate, abbreviated as MGC, is a progestin medication which was never marketed. It was developed in Russia in 2002. In animals, MGC shows 10-fold higher progestogenic activity compared to progesterone when both are administered via subcutaneous injection. In addition, MGC has no androgenic, anabolic, or estrogenic activity. The medication was suggested as a potential contraceptive and therapeutic agent.

20β-Dihydroprogesterone (20β-DHP), also known as 20β-hydroxyprogesterone (20β-OHP), is an endogenous metabolite of progesterone which is formed by 20β-hydroxysteroid dehydrogenase (20β-HSD). It is a progestogen similarly to progesterone, with about 20 to 50% of the progestogenic activity of progesterone. It can be converted by 20β-HSD into progesterone in the uterus. The effects of 20β-HSD on the uterus, mammary glands, and in maintaining pregnancy have been studied. The progestogenic activity of 20β-HSD has also been characterized in women.

Estradiol valerate/gestonorone caproate (EV/GC), known by the developmental code names SH-834 and SH-8.0834, is a high-dose combination medication of estradiol valerate (EV), an estrogen, and gestonorone caproate, a progestin, which was developed and studied by Schering in the 1960s and 1970s for potential use in the treatment of breast cancer in women but was ultimately never marketed. It contained 90 mg EV and 300 mg GC in each 3 mL of oil solution and was intended for use by intramuscular injection once a week. The combination has also been studied incidentally in the treatment of ovarian cancer.