 | |
| Clinical data | |
|---|---|
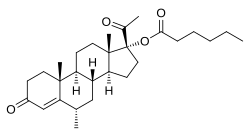
| Other names | MPC; Medroxyprogesterone capronate; Medroxyprogesterone hexanoate; 6α-Methyl-17α-hydroxyprogesterone hexanoate; 6α-Methyl-17α-hydroxypregn-4-ene-3,20-dione hexanoate |
| Routes of administration | Intramuscular injection |
| Drug class | Progestogen; Progestin; Progestogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H42O4 |
| Molar mass | 442.640 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Medroxyprogesterone caproate (MPC) is a progestin and a progestogen ester which was synthesized in 1958 but was never marketed. [1] [2] It has been confused with hydroxyprogesterone caproate (OHPC) and medroxyprogesterone acetate (MPA) in a number of publications. [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] In addition to MPA and OHPC, analogues of MPC include chlormadinone caproate, gestonorone caproate, megestrol caproate, and methenmadinone caproate.