 | |
| Clinical data | |
|---|---|
| Trade names | Finajet, Finaplix, others |
| Other names | RU-1697; Trenbolone 17β-acetate; 19-Nor-δ9,11-testosterone 17β-acetate; Estra-4,9,11-trien-17β-ol-3-one 17β-acetate |
| Routes of administration | Intramuscular |
| Drug class | Androgen; Anabolic steroid; Androgen ester; Progestogen |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | IM : 3 days [1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.030.380 |
| Chemical and physical data | |
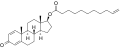
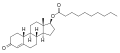
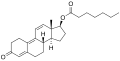
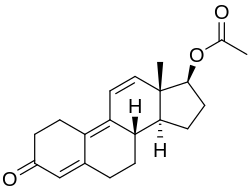
| Formula | C20H24O3 |
| Molar mass | 312.409 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Trenbolone acetate, sold under brand names such as Finajet and Finaplix among others, is an androgen and anabolic steroid (AAS) medication used in veterinary medicine, specifically to increase the profitability of livestock by promoting muscle growth in cattle. [2] [3] [4] [5] It is given by injection into muscle. [5] [2]
Contents
- Uses
- Veterinary uses
- Non-medical uses
- Medical uses
- Side effects
- Androgenic
- Hypogonadism
- Cardiovascular
- "Tren cough"
- Estrogenic and progestogenic
- Pharmacology
- Pharmacodynamics
- Pharmacokinetics
- Chemistry
- Structure–activity relationships
- History
- Society and culture
- Generic names
- Brand names
- Distribution and regulation
- Doping in sports
- References
- Further reading
- External links
Side effects of trenbolone acetate include symptoms of masculinization like acne, increased body hair growth, scalp hair loss, voice changes, and increased sexual desire. [5] The drug is a synthetic androgen and anabolic steroid [6] and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). [5] [2] [7] It has strong anabolic effects and highly androgenic effects, as well as potent progestogenic effects, and weak glucocorticoid effects. [5] [2] [7] [8] [9] Trenbolone acetate is an androgen ester and a short-lasting prodrug of trenbolone in the body.
Trenbolone acetate was discovered in 1963 and was introduced for veterinary use in the early 1970s. [5] [10] [11] In addition to its veterinary use, trenbolone acetate is used to improve physique and performance, for which purpose it is purchased from black market suppliers. [5] The drug is a controlled substance in many countries and so non-veterinary use is generally illicit. [5]