 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Synalar, others |
| AHFS/Drugs.com | Monograph Monograph |
| Pregnancy category |
|
| Routes of administration | Topical, ophthalmic intravitreal injection |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver, CYP3A4-mediated |
| Elimination half-life | 1.3 to 1.7 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.607 |
| Chemical and physical data | |
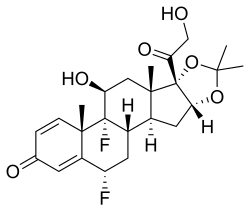
| Formula | C24H30F2O6 |
| Molar mass | 452.495 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fluocinolone acetonide is a fluorinated corticosteroid primarily used in dermatology to reduce skin inflammation and relieve itching. [2] It is a synthetic hydrocortisone derivative. The fluorine substitution at position 9 in the steroid nucleus greatly enhances its activity. It was first synthesized in 1959 in the Research Department of Syntex Laboratories S.A. Mexico City. [3] Preparations containing it were first marketed under the brand name Synalar.
Contents
Fluocinolone acetonide was also found to strongly potentiate TGF-β-associated chondrogenesis of bone marrow mesenchymal stem/progenitor cells, by increasing the levels of collagen type II by more than 100 fold compared to the widely used dexamethasone. [4]
Fluocinolone acetonide intravitreal implants have been used to treat non-infectious uveitis. A systematic review could not determine with any confidence whether fluocinolone acetonide implants are superior to standard of care treatment for uveitis. [5] A fluocinolone acetonide intravitreal implant with the brand name Iluvien is sold by biopharmaceutical company Alimera Sciences to treat diabetic macular edema. [6]
It was approved for medical use in 1961. [7]