Allergic conjunctivitis (AC) is inflammation of the conjunctiva due to allergy. Although allergens differ among patients, the most common cause is hay fever. Symptoms consist of redness, edema (swelling) of the conjunctiva, itching, and increased lacrimation. If this is combined with rhinitis, the condition is termed allergic rhinoconjunctivitis (ARC).

Desonide (INN) is a low-potency topical corticosteroid anti-inflammatory that has been available since the 1970s. It is primarily used to treat atopic dermatitis (eczema), seborrheic dermatitis, contact dermatitis and psoriasis in both adults and children. It has a fairly good safety profile and is available as a cream, ointment, lotion, and as a foam under the tradename Verdeso Foam. Other trade names for creams, lotions, and ointments include Tridesilon, DesOwen, Desonate. It is a group VI corticosteroid under US classification, the second least potent group.

Rimexolone is a glucocorticoid steroid used to treat inflammation in the eye. It is marketed as a 1% eye drop suspension under the trade name Vexol by Alcon Laboratories, but was discontinued in the US and other countries.

Loteprednol is a topical corticosteroid used to treat inflammations of the eye. It is marketed by Bausch and Lomb as Lotemax and Loterex.

Amcinonide is a topical glucocorticoid used to treat itching, redness and swelling associated with several dermatologic conditions such as atopic dermatitis and allergic contact dermatitis. Amcinonide can also be classified as a multi-functional small molecule corticosteroid, which has been approved by the FDA and is currently marketed as an ointment, lotion, or cream. It acts as both a transcription factor for responses to glucocorticoids and modulator for other transcription factors while also regulating phospholipase A2 activity.
Lebrikizumab (INN) is a humanized monoclonal antibody and an experimental immunosuppressive drug for the treatment of asthma that cannot be adequately controlled with inhalable glucocorticoids. The drug was created by Tanox under the name TNX-650, and a phase I clinical trial for refractory Hodgkin’s lymphoma had been performed when Genentech acquired Tanox in 2007. It has successfully completed a Phase II clinical trial for the treatment of asthma.

Prostaglandin D2 receptor 2 (DP2 or CRTH2) is a human protein encoded by the PTGDR2 gene and GPR44. DP2 has also been designated as CD294 (cluster of differentiation 294). It is a member of the class of prostaglandin receptors which bind with and respond to various prostaglandins. DP2 along with Prostaglandin DP1 receptor are receptors for prostaglandin D2 (PGD2). Activation of DP2 by PGD2 or other cognate receptor ligands has been associated with certain physiological and pathological responses, particularly those associated with allergy and inflammation, in animal models and certain human diseases.

Eltrombopag, sold under the brand name Promacta among others, is a medication used to treat thrombocytopenia and severe aplastic anemia. Eltrombopag is sold under the brand name Revolade outside the US and is marketed by Novartis. It is a thrombopoietin receptor agonist. It is taken by mouth.

Selective glucocorticoid receptor modulators (SEGRMs) and selective glucocorticoid receptor agonists (SEGRAs) formerly known as dissociated glucocorticoid receptor agonists (DIGRAs) are a class of experimental drugs designed to share many of the desirable anti-inflammatory, immunosuppressive, or anticancer properties of classical glucocorticoid drugs but with fewer side effects such as skin atrophy. Although preclinical evidence on SEGRAMs’ anti-inflammatory effects are culminating, currently, the efficacy of these SEGRAMs on cancer are largely unknown.
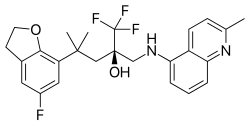
Lasmiditan, sold under the brand name Reyvow, is a medication used for the acute treatment of migraine with or without aura in adults. It is not useful for prevention. It is taken by mouth.

Alcaftadine is used to prevent eye irritation brought on by allergic conjunctivitis. It is a H1 histamine receptor antagonist.
Ixekizumab, sold under the brand name Taltz, is an injectable medication for the treatment of autoimmune diseases. Chemically, it is a form of a humanized monoclonal antibody. The substance acts by binding interleukin 17A and neutralizing it, reducing inflammation.
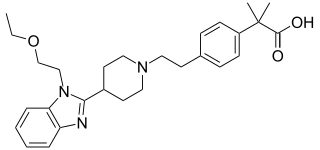
Bilastine is an antihistamine medication used to treat hives (urticaria) and inflammation of the eye (allergic conjunctivitis) caused by an allergy. It is a second-generation antihistamine and takes effect by selectively inhibiting the histamine H1 receptor, preventing these allergic reactions. Bilastine has an effectiveness similar to cetirizine, fexofenadine, and desloratadine. It was originally developed in Spain by FAES Farma.

Brexpiprazole, sold under the brand name Rexulti among others, is an atypical antipsychotic. It is a dopamine D2 receptor partial agonist and has been described as a "serotonin–dopamine activity modulator" (SDAM). The drug was approved by the U.S. Food and Drug Administration (FDA) on 10 July 2015, for the treatment of schizophrenia, and as an adjunctive treatment for depression. It has been designed to provide improved efficacy and tolerability (e.g., less akathisia, restlessness and/or insomnia) over established adjunctive treatments for major depressive disorder (MDD).
Dupilumab, sold under the brand name Dupixent, is a monoclonal antibody blocking interleukin 4 and interleukin 13, used for allergic diseases such as eczema, asthma and nasal polyps which result in chronic sinusitis. It is also used for the treatment of eosinophilic esophagitis and prurigo nodularis.

Oliceridine, sold under the brand name Olinvyk, is an opioid medication that is used for the treatment of moderate to severe acute pain in adults. It is given by intravenous (IV) injection.

Upadacitinib, sold under the brand name Rinvoq, is a Janus kinase (JAK) inhibitor medication for the treatment of moderately to severely active rheumatoid arthritis and psoriatic arthritis in adults where methotrexate did not work well or could not be tolerated. It was approved for medical use in the United States and in the European Union in 2019, and was developed by the biotech company AbbVie. On January 14, 2022 Rinvoq received FDA approval for treating treatment refractory atopic dermatitis.

Abrocitinib, sold under the brand name Cibinqo, is a Janus kinase inhibitor medication used for the treatment of atopic dermatitis (eczema). It was developed by Pfizer.

Tirzepatide, sold under the brand name Mounjaro, is an antidiabetic medication used for the treatment of type 2 diabetes. Tirzepatide is given by weekly subcutaneous injection.
Topical prednisolone is a type of glucocorticoid, mainly used in the ophthalmic pathway as eye drops in numerous eye conditions, such as corneal injuries caused by chemicals, burns and alien objects, inflammation of the eyes, mild to moderate non-infectious allergies, disorders of the eyelid, conjunctiva or sclera, ocular inflammation caused by operation and optic neuritis. Some side effects include glaucoma, blurring of vision, eye discomfort, impaired recovery of injured site, scarring of the optic nerve, clouding of lenses and urticaria. However, their prevalence is not known.