Electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element to attract shared electrons when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons.
Chemometrics is the science of extracting information from chemical systems by data-driven means. Chemometrics is inherently interdisciplinary, using methods frequently employed in core data-analytic disciplines such as multivariate statistics, applied mathematics, and computer science, in order to address problems in chemistry, biochemistry, medicine, biology and chemical engineering. In this way, it mirrors other interdisciplinary fields, such as psychometrics and econometrics.

A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is a document that lists information relating to occupational safety and health for the use of various substances and products. SDSs are a widely used type of fact sheet used to catalogue information on chemical species including chemical compounds and chemical mixtures. SDS information may include instructions for the safe use and potential hazards associated with a particular material or product, along with spill-handling procedures. The older MSDS formats could vary from source to source within a country depending on national requirements; however, the newer SDS format is internationally standardized.
Cheminformatics refers to the use of physical chemistry theory with computer and information science techniques—so called "in silico" techniques—in application to a range of descriptive and prescriptive problems in the field of chemistry, including in its applications to biology and related molecular fields. Such in silico techniques are used, for example, by pharmaceutical companies and in academic settings to aid and inform the process of drug discovery, for instance in the design of well-defined combinatorial libraries of synthetic compounds, or to assist in structure-based drug design. The methods can also be used in chemical and allied industries, and such fields as environmental science and pharmacology, where chemical processes are involved or studied.

Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) is a European Union regulation dating from 18 December 2006. REACH addresses the production and use of chemical substances, and their potential impacts on both human health and the environment. Its 849 pages took seven years to pass, and it has been described as the most complex legislation in the Union's history and the most important in 20 years. It is the strictest law to date regulating chemical substances and will affect industries throughout the world. REACH entered into force on 1 June 2007, with a phased implementation over the next decade. The regulation also established the European Chemicals Agency, which manages the technical, scientific and administrative aspects of REACH.

The Toxic Substances Control Act (TSCA) is a United States law, passed by the 94th United States Congress in 1976 and administered by the United States Environmental Protection Agency (EPA), that regulates chemicals not regulated by other U.S. federal statutes, including chemicals already in commerce and the introduction of new chemicals. When the TSCA was put into place, all existing chemicals were considered to be safe for use and subsequently grandfathered in. Its three main objectives are to assess and regulate new commercial chemicals before they enter the market, to regulate chemicals already existing in 1976 that posed an "unreasonable risk of injury to health or the environment", as for example PCBs, lead, mercury and radon, and to regulate these chemicals' distribution and use.
This is a list of the various reported boiling points for the elements, with recommended values to be used elsewhere on Wikipedia.
Registry of Toxic Effects of Chemical Substances (RTECS) is a database of toxicity information compiled from the open scientific literature without reference to the validity or usefulness of the studies reported. Until 2001 it was maintained by US National Institute for Occupational Safety and Health (NIOSH) as a freely available publication. It is now maintained by the private company BIOVIA or from several value-added resellers and is available only for a fee or by subscription.
PubChem is a database of chemical molecules and their activities against biological assays. The system is maintained by the National Center for Biotechnology Information (NCBI), a component of the National Library of Medicine, which is part of the United States National Institutes of Health (NIH). PubChem can be accessed for free through a web user interface. Millions of compound structures and descriptive datasets can be freely downloaded via FTP. PubChem contains multiple substance descriptions and small molecules with fewer than 100 atoms and 1,000 bonds. More than 80 database vendors contribute to the growing PubChem database.

KEGG is a collection of databases dealing with genomes, biological pathways, diseases, drugs, and chemical substances. KEGG is utilized for bioinformatics research and education, including data analysis in genomics, metagenomics, metabolomics and other omics studies, modeling and simulation in systems biology, and translational research in drug development.

The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) is an internationally agreed-upon standard managed by the United Nations that was set up to replace the assortment of hazardous material classification and labelling schemes previously used around the world. Core elements of the GHS include standardized hazard testing criteria, universal warning pictograms, and safety data sheets which provide users of dangerous goods relevant information with consistent organization. The system acts as a complement to the UN numbered system of regulated hazardous material transport. Implementation is managed through the UN Secretariat. Although adoption has taken time, as of 2017, the system has been enacted to significant extents in most major countries of the world. This includes the European Union, which has implemented the United Nations' GHS into EU law as the CLP Regulation, and United States Occupational Safety and Health Administration standards.
Chemical Entities of Biological Interest, also known as ChEBI, is a chemical database and ontology of molecular entities focused on 'small' chemical compounds, that is part of the Open Biomedical Ontologies (OBO) effort at the European Bioinformatics Institute (EBI). The term "molecular entity" refers to any "constitutionally or isotopically distinct atom, molecule, ion, ion pair, radical, radical ion, complex, conformer, etc., identifiable as a separately distinguishable entity". The molecular entities in question are either products of nature or synthetic products which have potential bioactivity. Molecules directly encoded by the genome, such as nucleic acids, proteins and peptides derived from proteins by proteolytic cleavage, are not as a rule included in ChEBI.
The MetaCyc database is one of the largest metabolic pathways and enzymes databases currently available. The data in the database is manually curated from the scientific literature, and covers all domains of life. MetaCyc has extensive information about chemical compounds, reactions, metabolic pathways and enzymes. The data have been curated from more than 58,000 publications.
ChemSpider is a freely accessible online database of chemicals owned by the Royal Society of Chemistry. It contains information on more than 100 million molecules from over 270 data sources, each of them receiving a unique identifier called ChemSpider Identifier.
Hazard statements form part of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). They are intended to form a set of standardized phrases about the hazards of chemical substances and mixtures that can be translated into different languages. As such, they serve the same purpose as the well-known R-phrases, which they are intended to replace.
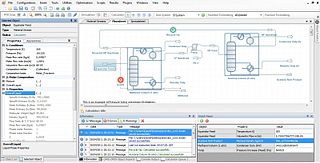
Process simulation is used for the design, development, analysis, and optimization of technical process of simulation of processes such as: chemical plant s, chemical processes, environmental systems, power stations, complex manufacturing operations, biological processes, and similar technical functions.
Molecular communications systems use the presence or absence of a selected type of molecule to digitally encode messages. The molecules are delivered into communications media such as air and water for transmission. The technique also is not subject to the requirement of using antennas that are sized to a specific ratio of the wavelength of the signal. Molecular communication signals can be made biocompatible and require very little energy.

The CompTox Chemicals Dashboard is a freely accessible online database created and maintained by the U.S. Environmental Protection Agency (EPA). The database provides access to multiple types of data including physicochemical properties, environmental fate and transport, exposure, usage, in vivo toxicity, and in vitro bioassay. EPA and other scientists use the data and models contained within the dashboard to help identify chemicals that require further testing and reduce the use of animals in chemical testing. The Dashboard is also used to provide public access to information from EPA Action Plans, e.g. around perfluorinated alkylated substances.







