
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids and sex steroids. Within those two classes are five types according to the receptors to which they bind: glucocorticoids and mineralocorticoids and androgens, estrogens, and progestogens. Vitamin D derivatives are a sixth closely related hormone system with homologous receptors. They have some of the characteristics of true steroids as receptor ligands.

Cortisol is a steroid hormone, in the glucocorticoid class of hormones and a stress hormone. When used as a medication, it is known as hydrocortisone.

Glucocorticoids are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every vertebrate animal cell. The name "glucocorticoid" is a portmanteau and is composed from its role in regulation of glucose metabolism, synthesis in the adrenal cortex, and its steroidal structure.

Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances. The primary mineralocorticoid is aldosterone.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.
11β-Hydroxysteroid dehydrogenase enzymes catalyze the conversion of inert 11 keto-products (cortisone) to active cortisol, or vice versa, thus regulating the access of glucocorticoids to the steroid receptors.

The glucocorticoid receptor also known as NR3C1 is the receptor to which cortisol and other glucocorticoids bind.

Apparent mineralocorticoid excess is an autosomal recessive disorder causing hypertension, hypernatremia and hypokalemia. It results from mutations in the HSD11B2 gene, which encodes the kidney isozyme of 11β-hydroxysteroid dehydrogenase type 2. In an unaffected individual, this isozyme inactivates circulating cortisol to the less active metabolite cortisone. The inactivating mutation leads to elevated local concentrations of cortisol in the aldosterone sensitive tissues like the kidney. Cortisol at high concentrations can cross-react and activate the mineralocorticoid receptor due to the non-selectivity of the receptor, leading to aldosterone-like effects in the kidney. This is what causes the hypokalemia, hypertension, and hypernatremia associated with the syndrome. Patients often present with severe hypertension and end-organ changes associated with it like left ventricular hypertrophy, retinal, renal and neurological vascular changes along with growth retardation and failure to thrive. In serum both aldosterone and renin levels are low.

Eplerenone, sold under the brand name Inspra, is an aldosterone antagonist type of potassium-sparing diuretic that is used to treat chronic heart failure and high blood pressure, particularly for patients with resistant hypertension due to elevated aldosterone. It is a steroidal antimineralocorticoid of the spirolactone group and a selective aldosterone receptor antagonist (SARA). Eplerenone is more selective than spironolactone at the mineralocorticoid receptor relative to binding at androgen, progestogen, glucocorticoid, or estrogen receptors.

Tibolone, sold under the brand name Livial among others, is a medication which is used in menopausal hormone therapy and in the treatment of postmenopausal osteoporosis and endometriosis. The medication is available alone and is not formulated or used in combination with other medications. It is taken by mouth.

Corticosteroid 11-β-dehydrogenase isozyme 2 also known as 11-β-hydroxysteroid dehydrogenase 2 is an enzyme that in humans is encoded by the HSD11B2 gene.

The mineralocorticoid receptor, also known as the aldosterone receptor or nuclear receptor subfamily 3, group C, member 2, (NR3C2) is a protein that in humans is encoded by the NR3C2 gene that is located on chromosome 4q31.1-31.2.
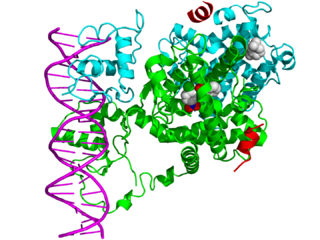
In the field of molecular biology, nuclear receptors are a class of proteins responsible for sensing steroids, thyroid hormones, vitamins, and certain other molecules. These intracellular receptors work with other proteins to regulate the expression of specific genes thereby controlling the development, homeostasis, and metabolism of the organism.

FK506 binding protein 5, also known as FKBP5, is a protein which in humans is encoded by the FKBP5 gene.

Selective glucocorticoid receptor modulators (SEGRMs) and selective glucocorticoid receptor agonists (SEGRAs) formerly known as dissociated glucocorticoid receptor agonists (DIGRAs) are a class of experimental drugs designed to share many of the desirable anti-inflammatory, immunosuppressive, or anticancer properties of classical glucocorticoid drugs but with fewer side effects such as skin atrophy. Although preclinical evidence on SEGRAMs’ anti-inflammatory effects are culminating, currently, the efficacy of these SEGRAMs on cancer are largely unknown.

Trimegestone, sold under the brand names Ondeva and Totelle among others, is a progestin medication which is used in menopausal hormone therapy and in the prevention of postmenopausal osteoporosis. It was also under development for use in birth control pills to prevent pregnancy, but ultimately was not marketed for this purpose. The medication is available alone or in combination with an estrogen. It is taken by mouth.

Mexrenone is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed. It is the lactonic form of mexrenoic acid (mexrenoate), and mexrenoate potassium (SC-26714), the potassium salt of mexrenoic acid, also exists. In addition to the mineralocorticoid receptor, mexrenone also binds to the glucocorticoid, androgen, and progesterone receptors. Relative to spironolactone, it has markedly reduced antiandrogen activity. Eplerenone is the 9-11α-epoxy analogue of mexrenone.
Membrane mineralocorticoid receptors (mMRs) or membrane aldosterone receptors are a group of receptors which bind and are activated by mineralocorticoids such as aldosterone. Unlike the classical nuclear mineralocorticoid receptor (MR), which mediates its effects via genomic mechanisms, mMRs are cell surface receptors which rapidly alter cell signaling via modulation of intracellular signaling cascades. The identities of the mMRs have yet to be fully elucidated, but are thought to include membrane-associated classical MRs as well as yet-to-be-characterized G protein-coupled receptors (GPCRs). Rapid effects of aldosterone were found not be reversed by the MR antagonist spironolactone, indicating additional receptors besides just the classical MR. It has been estimated that as much as 50% of the rapid actions of aldosterone are mediated by mMRs that are not the classical MR, based on findings of insensitivity to classical mR antagonists.

RU-59063 is a nonsteroidal androgen or selective androgen receptor modulator (SARM) which was first described in 1994 and was never marketed. It was originally thought to be a potent antiandrogen, but subsequent research found that it actually possesses dose-dependent androgenic activity, albeit with lower efficacy than dihydrotestosterone (DHT). The drug is an N-substituted arylthiohydantoin and was derived from the first-generation nonsteroidal antiandrogen (NSAA) nilutamide. The second-generation NSAAs enzalutamide, RD-162, and apalutamide were derived from RU-59063.

Miricorilant is a small molecule that works as a selective glucocorticoid receptor modulator and mineralcorticoid receptor antagonist. It was developed by Corcept Therapeutics for nonalcoholic steatohepatitis and weight gain associated with atypical antipsychotics.

















