
The World Health Organization (WHO) is a specialized agency of the United Nations responsible for international public health. It is headquartered in Geneva, Switzerland, and has six regional offices and 150 field offices worldwide.
Health has a variety of definitions, which have been used for different purposes over time. In general, it refers to physical and emotional well-being, especially that associated with normal functioning of the human body, absent of disease, pain, or injury.

Diethylcarbamazine is a medication used in the treatment of filariasis including lymphatic filariasis, tropical pulmonary eosinophilia, and loiasis. It may also be used for prevention of loiasis in those at high risk. While it has been used for onchocerciasis, ivermectin is preferred. It is taken by mouth.
Human body weight is a person's mass or weight.

Cloxacillin is an antibiotic useful for the treatment of several bacterial infections. This includes impetigo, cellulitis, pneumonia, septic arthritis, and otitis externa. It is not effective for methicillin-resistant Staphylococcus aureus (MRSA). It can be used by mouth and by injection.

Amoxicillin/clavulanic acid, also known as co-amoxiclav or amox-clav, sold under the brand name Augmentin, among others, is an antibiotic medication used for the treatment of a number of bacterial infections. It is a combination consisting of amoxicillin, a β-lactam antibiotic, and potassium clavulanate, a β-lactamase inhibitor. It is specifically used for otitis media, streptococcal pharyngitis, pneumonia, cellulitis, urinary tract infections, and animal bites. It is taken by mouth or by injection into a vein.

The World Health Assembly (WHA) is the forum through which the World Health Organization (WHO) is governed by its 194 member states. It is the world's highest health policy setting body and is composed of health ministers from member states.

Calamine, also known as calamine lotion, is a medication made from powdered calamine mineral that is used to treat mild itchiness. Conditions treated include sunburn, insect bites, poison ivy, poison oak, and other mild skin conditions. It may also help dry out secretions resulting from skin irritation. It is applied on the skin as a cream or lotion.

Procarbazine is a chemotherapy medication used for the treatment of Hodgkin's lymphoma and brain cancers. For Hodgkin's it is often used together with chlormethine, vincristine, and prednisone while for brain cancers such as glioblastoma multiforme it is used with lomustine and vincristine. It is typically taken by mouth.
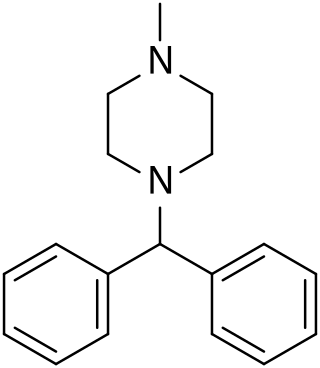
Cyclizine, sold under a number of brand names, is a medication used to treat and prevent nausea, vomiting and dizziness due to motion sickness or vertigo. It may also be used for nausea after general anaesthesia or that which developed from opioid use. It is taken by mouth, in the rectum, or injected into a vein.
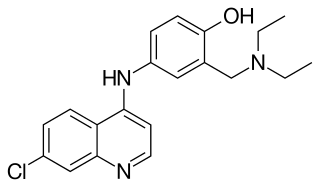
Amodiaquine (ADQ) is a medication used to treat malaria, including Plasmodium falciparum malaria when uncomplicated. It is recommended to be given with artesunate to reduce the risk of resistance. Due to the risk of rare but serious side effects, it is not generally recommended to prevent malaria. Though, the World Health Organization (WHO) in 2013 recommended use for seasonal preventive in children at high risk in combination with sulfadoxine and pyrimethamine.
Translators without Borders (TWB) is a non-profit organization set up to provide translation services for humanitarian non-profits. It was established in 2010 as a sister organization of Traducteurs Sans Frontières, founded in 1993 by Lori Thicke and Ros Smith-Thomas. As of 2022, it had over 100,000 members. TWB's objective is to address language disparities that impede crucial humanitarian efforts. They aim to accomplish this by facilitating collaboration between non-profit humanitarian entities and a volunteer community of translators.
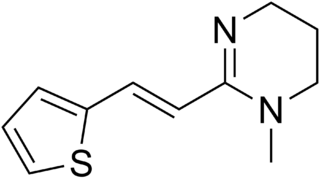
Pyrantel is a medication used to treat a number of parasitic worm infections. This includes ascariasis, hookworm infections, enterobiasis, trichostrongyliasis, and trichinellosis. It is taken by mouth.
Below are two tables which report the average adult human height by country or geographical region. With regard to the first table, original studies and sources should be consulted for details on methodology and the exact populations measured, surveyed, or considered. With regard to the second table, these estimated figures for adult human height for said countries and territories in 2019 and the declared sources may conflict with the findings of the first table.

Tedros Adhanom Ghebreyesus is an Ethiopian public health official, researcher, diplomat, and the Director-General of the World Health Organization since 2017. He is the first African to become WHO Director-General, receiving an endorsement for the role by the African Union. Tedros played a role in the response to the Ebola virus epidemic, the COVID-19 pandemic, and the 2022–2023 mpox outbreak.

Umeclidinium bromide, sold under the brand name Incruse Ellipta, is a long-acting muscarinic antagonist approved for the maintenance treatment of chronic obstructive pulmonary disease (COPD). It is also approved for this indication in combination with vilanterol and also as a triple-therapy combination as fluticasone furoate/umeclidinium bromide/vilanterol.












