
Lasofoxifene, sold under the brand name Fablyn, is a nonsteroidal selective estrogen receptor modulator (SERM) which is marketed by Pfizer in Lithuania and Portugal for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND). It also appears to have had a statistically significant effect of reducing breast cancer in women according to a study published in The Journal of the National Cancer Institute.

Telapristone, as telapristone acetate, is a synthetic, steroidal selective progesterone receptor modulator (SPRM) related to mifepristone which is under development by Repros Therapeutics for the treatment of breast cancer, endometriosis, and uterine fibroids. It was originally developed by the National Institutes of Health (NIH), and, as of 2017, is in phase II clinical trials for the aforementioned indications. In addition to its activity as an SPRM, the drug also has some antiglucocorticoid activity.

Selective androgen receptor modulators (SARMs) are a class of drugs that selectively activate the androgen receptor in specific tissues, promoting muscle and bone growth while having less effect on male reproductive tissues like the prostate gland.
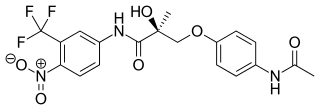
Andarine is a selective androgen receptor modulator (SARM) which was developed by GTX, Inc for the treatment of conditions such as muscle wasting, osteoporosis, and benign prostatic hypertrophy (BPH), using the nonsteroidal antiandrogen bicalutamide as a lead compound. Development of andarine for all indications has been discontinued, in favor of the structurally related and improved compound enobosarm.

Selective glucocorticoid receptor modulators (SEGRMs) and selective glucocorticoid receptor agonists (SEGRAs) formerly known as dissociated glucocorticoid receptor agonists (DIGRAs) are a class of experimental drugs designed to share many of the desirable anti-inflammatory, immunosuppressive, or anticancer properties of classical glucocorticoid drugs but with fewer side effects such as skin atrophy. Although preclinical evidence on SEGRAMs’ anti-inflammatory effects are culminating, currently, the efficacy of these SEGRAMs on cancer are largely unknown.

Mapracorat is an anti-inflammatory drug belonging to the experimental class of selective glucocorticoid receptor agonists (SEGRAs). It is in clinical trials for the topical treatment of atopic dermatitis, inflammation following cataract surgery, and allergic conjunctivitis. Preliminary investigation for the treatment of keratoconjunctivitis sicca has been conducted in cellular models.
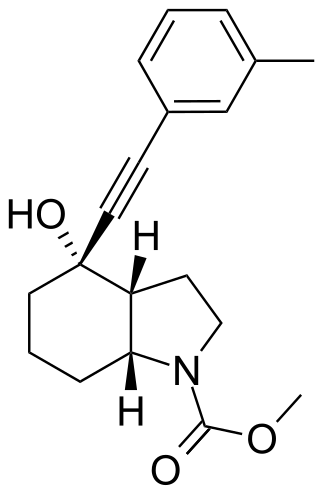
Mavoglurant (developmental code name AFQ-056) is an experimental drug candidate for the treatment of fragile X syndrome and other conditions. It exerts its effect as an antagonist of the metabotropic glutamate receptor 5 (mGluR5).

Abediterol is a once-daily experimental drug candidate for the treatment of asthma and chronic obstructive pulmonary disease (COPD), but it has never been marketed.

Samidorphan is an opioid antagonist that in the form of olanzapine/samidorphan is used in the treatment of schizophrenia and bipolar disorder. Samidorphan reduces the weight gain associated with olanzapine. Samidorphan is taken by mouth.

Acolbifene is a nonsteroidal selective estrogen receptor modulator (SERM) which, as of 2015, is in phase III clinical trials for the treatment of breast cancer.

Seviteronel is an experimental cancer medication which is under development by Viamet Pharmaceuticals and Innocrin Pharmaceuticals for the treatment of prostate cancer and breast cancer. It is a nonsteroidal CYP17A1 inhibitor and works by inhibiting the production of androgens and estrogens in the body. As of July 2017, seviteronel is in phase II clinical trials for both prostate cancer and breast cancer. In January 2016, it was designated fast-track status by the United States Food and Drug Administration for prostate cancer. In April 2017, seviteronel received fast-track designation for breast cancer as well.

Vosilasarm, also known by the development codes RAD140 and EP0062 and by the black-market name Testolone or Testalone, is a selective androgen receptor modulator (SARM) which is under development for the treatment of hormone-sensitive breast cancer. It is specifically under development for the treatment of androgen receptor-positive, estrogen receptor-negative, HER2-negative advanced breast cancer. Vosilasarm was also previously under development for the treatment of sarcopenia, osteoporosis, and weight loss due to cancer cachexia, but development for these indications was discontinued. The drug is taken by mouth.

Droloxifene, also known as 3-hydroxytamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed originally in Germany and later in Japan for the treatment of breast cancer, osteoporosis in men and postmenopausal women, and cardiovascular disorders but was abandoned and never marketed. It reached phase II and phase III clinical trials for these indications before development was discontinued in 2000. The drug was found to be significantly less effective than tamoxifen in the treatment of breast cancer in two phase III clinical trials.

Fispemifene is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed for the treatment of male hypogonadism but was abandoned and never marketed. It reached phase II clinical trials for this indication before development was terminated in March 2016. The drug failed to achieve statistical significance on key effectiveness endpoints in clinical trials and was discontinued by its developer for strategic reasons.

Pipendoxifene (INN) is a nonsteroidal selective estrogen receptor modulator (SERM) that was under development by Ligand Pharmaceuticals and Wyeth-Ayerst Laboratories for the treatment of breast cancer but was not marketed. It is a member of the 2-phenylindole group of SERMs and is structurally related to zindoxifene and the marketed bazedoxifene. The drug reached phase II clinical trials before its development was discontinued. It was synthesized at the same time as bazedoxifene and was intended as a backup drug for bazedoxifene, only to be developed further if bazedoxifene had failed in clinical trials. No further development was reported after 2002 and it was formally announced that development had been terminated in November 2005.

Irosustat is an orally active, irreversible, nonsteroidal inhibitor of steroid sulfatase (STS) and member of the aryl sulfamate ester class of drugs that was under development by Sterix Ltd and Ipsen for the treatment of hormone-sensitive cancers such as breast cancer, prostate cancer, and endometrial cancer but has not yet been marketed. The drug was first designed and synthesized in the group of Professor Barry V L Potter at the Department of Pharmacy & Pharmacology, University of Bath, working together with Professor Michael J. Reed at Imperial College, London and its initial development was undertaken through the university spin-out company Sterix Ltd and overseen by Cancer Research UK (CRUK). Results of the "first-in-class" clinical trial in breast cancer of an STS inhibitor in humans were published in 2006 and dose optimisation studies and further clinical data have been reported.
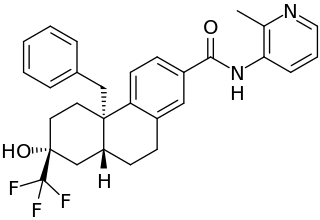
Dagrocorat is a nonsteroidal but steroid-like selective glucocorticoid receptor modulator (SGRM) which was under development for the treatment of rheumatoid arthritis but was never marketed. It is described as a partial agonist and "dissociable" agonist of the glucocorticoid receptor. The drug reached phase I clinical trials prior to the discontinuation of its development. The C2α dihydrogen phosphate ester of dagrocorat, fosdagrocorat, was also under investigation, but its development was terminated as well.
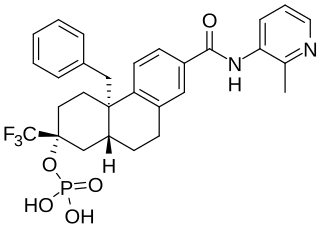
Fosdagrocorat is a nonsteroidal but steroid-like selective glucocorticoid receptor modulator (SGRM) which was under development for the treatment of rheumatoid arthritis but was never marketed. It is the C2 dihydrogen phosphate ester of dagrocorat, and acts as a prodrug of dagrocorat with improved pharmacokinetics. The drug reached phase II clinical trials prior to the discontinuation of its development.

Relacorilant is an antiglucocorticoid which is under development by Corcept Therapeutics for the treatment of Cushing's syndrome. It is also under development for the treatment of solid tumors and alcoholism. The drug is a nonsteroidal compound and acts as an antagonist of the glucocorticoid receptor. As of December 2017, it is in phase II clinical trials for Cushing's syndrome and phase I/II clinical studies for solid tumors, while the clinical phase for alcoholism is unknown.

EM-5854 is a steroidal antiandrogen which was under development by Endoceutics, Inc. for the treatment of prostate cancer. It was first described in a patent in 2008, and was further characterized in 2012. EM-5854 reached phase I/II clinical trials for the treatment of prostate cancer but development was discontinued in March 2019.



















