Dermatitis is a term used for different types of skin inflammation, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened. The area of skin involved can vary from small to covering the entire body. Dermatitis is also called eczema but the same term is often used for the most common type of skin inflammation, atopic dermatitis.

Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involved in a wide range of physiological processes, including stress response, immune response, and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels, and behavior.

Pimecrolimus is an immunosuppressant drug of the calcineurin inhibitor class used in the treatment of atopic dermatitis (eczema).
Antipruritics, abirritants, or anti-itch drugs, are medications that inhibit the itching often associated with sunburns, allergic reactions, eczema, psoriasis, chickenpox, fungal infections, insect bites and stings like those from mosquitoes, fleas, and mites, and contact dermatitis and urticaria caused by plants such as poison ivy or stinging nettle. It can also be caused by chronic kidney disease and related conditions.

Clobetasol propionate is a corticosteroid used to treat skin conditions such as eczema, contact dermatitis, seborrheic dermatitis, steroid responsive dermatosis, and psoriasis.

Desonide (INN) is a low-potency topical corticosteroid anti-inflammatory that has been available since the 1970s. It is primarily used to treat atopic dermatitis (eczema), seborrheic dermatitis, contact dermatitis and psoriasis in both adults and children. It has a fairly good safety profile and is available as a cream, ointment, lotion, and as a foam under the tradename Verdeso Foam. Other trade names for creams, lotions, and ointments include Tridesilon, DesOwen, Desonate. It is a group VI corticosteroid under US classification, the second least potent group.

Betamethasone dipropionate is a glucocorticoid steroid with anti-inflammatory and immunosuppressive properties. It is applied as a topical cream, ointment, lotion or gel (Diprolene) to treat itching and other skin conditions such as eczema. Minor side effects include dry skin and mild, temporary stinging when applied. Betamethasone dipropionate is a "super high potency" corticosteroid used to treat inflammatory skin conditions such as dermatitis, eczema and psoriasis. It is a synthetic analog of the adrenal corticosteroids. Although its exact mechanism of action is not known, it is effective when applied topically to cortico-responsive inflammatory dermatoses. It is available as a generic medication.

Calcipotriol, also known as calcipotriene, is a synthetic derivative of calcitriol, a form of vitamin D. It is used in the treatment of psoriasis. It is safe for long-term application in psoriatic skin conditions.

Perioral dermatitis, also known as periorificial dermatitis, is a common type of inflammatory skin rash. Symptoms include multiple small (1–2 mm) bumps and blisters sometimes with background redness and scale, localized to the skin around the mouth and nostrils. Less commonly, the eyes and genitalia may be involved. It can be persistent or recurring, and resembles particularly rosacea and to some extent acne and allergic dermatitis. The term "dermatitis" is a misnomer because this is not an eczematous process.
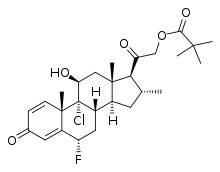
Alclometasone is a synthetic corticosteroid for topical dermatologic use, possessing anti-inflammatory, antipruritic, and vasoconstrictive properties.

Diflorasone diacetate is a topical steroid that comes in the form of a cream. It is manufactured by E. Fougera & Co. and is used as an anti-inflammatory and anti-itching agent, like other topical corticosteroids. It is prescribed for psoriasis and atopic dermatitis, among other conditions. With respect to potency, it is regarded as a Class I corticosteroid [of classes I – VII] in the United States.

Amcinonide is a topical glucocorticoid used to treat itching, redness and swelling associated with several dermatologic conditions such as atopic dermatitis and allergic contact dermatitis. Amcinonide can also be classified as a multi-functional small molecule corticosteroid, which has been approved by the FDA and is currently marketed as an ointment, lotion, or cream. It acts as both a transcription factor for responses to glucocorticoids and modulator for other transcription factors while also regulating phospholipase A2 activity.

Halometasone is a potent synthetic tri-halogenated corticosteroid for topical application possessing pronounced anti-inflammatory, antiexudative, antiepidermoplastic, antiallergic, and antipruritic properties. It has been approved in many European countries including Spain, Germany, Switzerland, Austria, Netherlands, Belgium, and Portugal and other regions such as China, Hong Kong, Turkey, Israel, South Africa and India.
In medicine, a finger tip unit (FTU) is defined as the amount of ointment, cream or other semi-solid dosage form expressed from a tube with a 5 mm diameter nozzle, applied from the distal skin-crease to the tip of the index finger of an adult. The "distal skin-crease" is the skin crease over the joint nearest the end of the finger. One FTU is enough to treat an area of skin twice the size of the flat of an adult's hand with the fingers together, i.e. a "handprint". Two FTUs are approximately equivalent to 1 g of topical steroid.
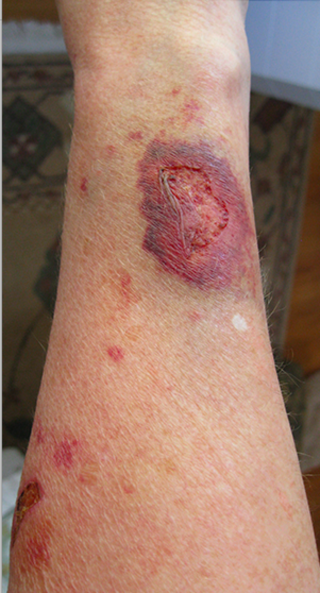
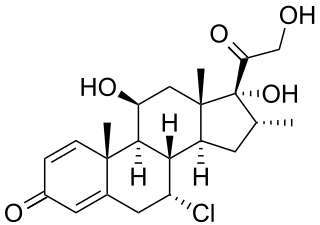
Steroid-induced skin atrophy is thinning of the skin as a result of prolonged exposure to topical steroids. In people with psoriasis using topical steroids it occurs in up to 5% of people after a year of use. Intermittent use of topical steroids for atopic dermatitis is safe and does not cause skin thinning.
Topical steroids are the topical forms of corticosteroids. Topical steroids are the most commonly prescribed topical medications for the treatment of rash and eczema. Topical steroids have anti-inflammatory properties and are classified based on their skin vasoconstrictive abilities. There are numerous topical steroid products. All the preparations in each class have the same anti-inflammatory properties but essentially differ in base and price.

Calcipotriol/betamethasone dipropionate, sold under the brand name Taclonex among others, is a fixed-dose combination medication of the synthetic vitamin D3 analog calcipotriol (also known as calcipotriene) and the synthetic corticosteroid betamethasone dipropionate for the treatment of plaque psoriasis. It is used in the form of ointment, topical suspension, gel, aerosol, and foam.

Topical steroid withdrawal, also known as red burning skin and steroid dermatitis, has been reported in people who apply topical steroids for 2 weeks or longer and then discontinue use. Symptoms affect the skin and include redness, a burning sensation, and itchiness, which may then be followed by peeling.

Topical glucocorticoids are the topical forms of glucocorticoids. Topical glucocorticoids are used in the treatment of many skin conditions. They provide anti-inflammatory, antimitotic, and immune-system suppressing actions through various mechanisms.

Topical hydrocortisone is a drug under the class of corticosteroids, which is used for the treatment of skin inflammation, itchiness and allergies. Some examples include insect bites, dermatitis and rash.