 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nasonex, Asmanex, Elocon, others [1] |
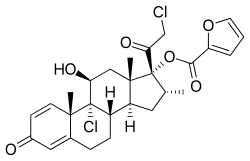
| Other names | LAS-41002, 9α,21-Dichloro-11β,17α-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 17α-(2-furoate) |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical, inhalation (nasal spray) |
| Drug class | Corticosteroid; Glucocorticoid |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Nasal spray is virtually undetectable in plasma; but systemic availability is comparable to fluticasone [9] |
| Protein binding | 98% to 99% |
| Metabolism | Liver |
| Elimination half-life | 5.8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.125.600 |
| Chemical and physical data | |
| Formula | C22H28Cl2O4 for mometasone C27H30O6Cl2 as furoate |
| 3D model (JSmol) |
|
| |
| |
| | |
Mometasone, and its derivate mometasone furoate, are steroids (specifically, glucocorticoids) medications used to treat certain skin conditions, hay fever, and asthma. [10] [11] [12] Specifically they are used to prevent rather than treat asthma attacks. [10] They can be applied to the skin, inhaled, or used in the nose. [10] [11] [12] Currently only mometasone furoate is used in medical products. [13]
Contents
- Medical uses
- Asthma
- Contraindications
- Side effects
- Pharmacology
- Pharmacodynamics
- Pharmacokinetics
- Mometasone
- Society and culture
- Availability
- Combinations
- References
Common side effects when used for asthma include headache, sore throat, and thrush. [10] It is therefore recommended to rinse the mouth after use. [10] Long-term use may increase the risk for glaucoma and cataracts. [10] Common side effects when used in the nose include upper respiratory tract infections and nose bleeds. [12] Common side effects when applied on the skin include acne, skin atrophy, and itchiness. [11] It works by decreasing inflammation. [10]
Mometasone furoate was patented in 1981 and came into medical use in 1987. [14] It is on the World Health Organization's List of Essential Medicines [15] and is available as a generic medication. [16] [17] In 2023, it was the 272nd most commonly prescribed medication in the United States, with more than 800,000 prescriptions. [18] [19]
