An antiemetic is a drug that is effective against vomiting and nausea. Antiemetics are typically used to treat motion sickness and the side effects of opioid analgesics, general anaesthetics, and chemotherapy directed against cancer. They may be used for severe cases of gastroenteritis, especially if the patient is dehydrated.

Ertapenem, sold under the brand name Invanz, is a carbapenem antibiotic medication used for the treatment of infections of the abdomen, the lungs, the upper part of the female reproductive system, and the diabetic foot.

Ondansetron, sold under the brand name Zofran among others, is a medication used to prevent nausea and vomiting caused by cancer chemotherapy, radiation therapy, migraines or surgery. It is also effective for treating gastroenteritis. It can be given orally, intramuscularly, or intravenously.

Aprepitant, sold under the brand name Emend among others, is a medication used to prevent chemotherapy-induced nausea and vomiting and to prevent postoperative nausea and vomiting. It may be used together with ondansetron and dexamethasone. It is taken by mouth or administered by intravenous injection.

Caspofungin is a lipopeptide antifungal drug from Merck & Co., Inc.. It is a member of a class of antifungals termed the echinocandins. It works by inhibiting the enzyme (1→3)-β-D-glucan synthase and thereby disturbing the integrity of the fungal cell wall.

Piperacillin/tazobactam, sold under the brand name Tazocin among others, is a combination medication containing the antibiotic piperacillin and the β-lactamase inhibitor tazobactam. The combination has activity against many Gram-positive and Gram-negative bacteria including Pseudomonas aeruginosa. It is used to treat pelvic inflammatory disease, intra-abdominal infection, pneumonia, cellulitis, and sepsis. It is given by injection into a vein.

Ganciclovir, sold under the brand name Cytovene among others, is an antiviral medication used to treat cytomegalovirus (CMV) infections.

Dolasetron (trade name Anzemet) is a serotonin 5-HT3 receptor antagonist used to treat nausea and vomiting following chemotherapy. Its main effect is to reduce the activity of the vagus nerve, which is a nerve that activates the vomiting center in the medulla oblongata. It does not have much antiemetic effect when symptoms are due to motion sickness. This drug does not have any effect on dopamine receptors or muscarinic receptors.

Droperidol is an antidopaminergic drug used as an antiemetic and as an antipsychotic. Droperidol is also often used as a rapid sedative in intensive-care treatment, and where "agitation aggression or violent behavior" are present.

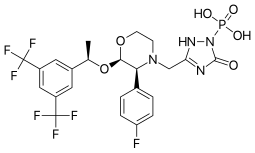
Palonosetron, sold under the brand name Aloxi, is a medication used for the prevention and treatment of chemotherapy-induced nausea and vomiting (CINV). It is a 5-HT3 antagonist.

Isavuconazonium, sold under the brand name Cresemba, is a systemic antifungal medication of the triazole class which is used to treat invasive aspergillosis and mucormycosis. It is used as the sulfate. It is taken by mouth or given via injection into a vein.
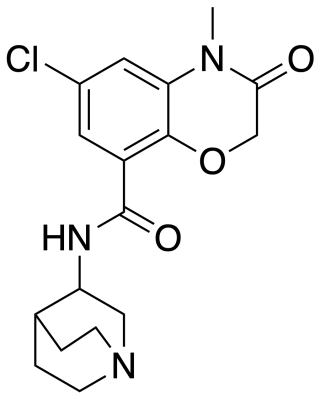
Azasetron is an antiemetic which acts as a 5-HT3 receptor antagonist, pKi = 9.27 It is used in the management of nausea and vomiting induced by cancer chemotherapy (such as cisplatin chemotherapy). Azasetron hydrochloride is given in a usual dose of 10 mg once daily by mouth or intravenously. It is approved for marketing in Japan, and marketed exclusively by Torii Pharmaceutical Co., Ltd. under the trade names "Serotone I.V. Injection 10 mg" and "Serotone Tablets 10 mg". Pharmacokinetics data from S. Tsukagoshi.

Maribavir, sold under the brand name Livtencity, is an antiviral medication that is used to treat post-transplant cytomegalovirus (CMV). Maribavir is a cytomegalovirus pUL97 kinase inhibitor that works by preventing the activity of human cytomegalovirus enzyme pUL97, thus blocking virus replication.

Teduglutide, sold under the brand names Revestive (EU) and Gattex (US), is a 33-membered polypeptide and glucagon-like peptide-2 (GLP-2) analog that is used for the treatment of short bowel syndrome. It works by promoting mucosal growth and possibly restoring gastric emptying and secretion. It was approved in both the European Union and the United States in 2012.
Netupitant/palonosetron, sold under the brand name Akynzeo, is a fixed-dose combination medication used for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. It is marketed and distributed by Helsinn Therapeutics. Netupitant is an NK1 receptor antagonist and palonosetron is a 5-HT3 receptor antagonist.

Sacituzumab govitecan, sold under the brand name Trodelvy by Gilead Sciences, is a Trop-2-directed antibody and topoisomerase inhibitor drug conjugate used for the treatment of metastatic triple-negative breast cancer and metastatic urothelial cancer.

Rolapitant (INN, trade name Varubivə-ROO-bee in the US and Varuby in the European Union) is a drug originally developed by Schering-Plough and licensed for clinical development by Tesaro, which acts as a selective NK1 receptor antagonist (antagonist for the NK1 receptor). It has been approved as a medication for the treatment of chemotherapy-induced nausea and vomiting (CINV) after clinical trials showed it to have similar or improved efficacy and some improvement in safety over existing drugs for this application.
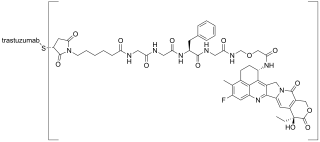
Trastuzumab deruxtecan, sold under the brand name Enhertu, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the topoisomerase I inhibitor deruxtecan. It is licensed for the treatment of breast cancer or gastric or gastroesophageal adenocarcinoma. Trastuzumab binds to and blocks signaling through epidermal growth factor receptor 2 (HER2/neu) on cancers that rely on it for growth. Additionally, once bound to HER2 receptors, the antibody is internalized by the cell, carrying the bound deruxtecan along with it, where it interferes with the cell's ability to make DNA structural changes and replicate its DNA during cell division, leading to DNA damage when the cell attempts to replicate itself, destroying the cell.
Vonicog alfa, sold under the brand names Vonvendi and Veyvondi, is a medication used to control bleeding in adults with von Willebrand disease. It is a recombinant von Willebrand factor.
Ciltacabtagene autoleucel, sold under the brand name Carvykti, is an anti-cancer medication used to treat multiple myeloma. Ciltacabtagene autoleucel is a BCMA -directed genetically modified autologous chimeric antigen receptor (CAR) T-cell therapy. Each dose is customized using the recipient's own T-cells, which are collected and genetically modified, and infused back into the recipient.