Paroxetine, sold under the brand names Paxil and Seroxat among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive-compulsive disorder, panic disorder, social anxiety disorder, posttraumatic stress disorder, generalized anxiety disorder and premenstrual dysphoric disorder. It has also been used in the treatment of premature ejaculation and hot flashes due to menopause. It is taken by mouth.
An antiemetic is a drug that is effective against vomiting and nausea. Antiemetics are typically used to treat motion sickness and the side effects of opioid analgesics, general anaesthetics, and chemotherapy directed against cancer. They may be used for severe cases of gastroenteritis, especially if the patient is dehydrated.

Ondansetron, sold under the brand name Zofran among others, is a medication used to prevent nausea and vomiting caused by cancer chemotherapy, radiation therapy, or surgery. It is also effective for treating gastroenteritis. It can be given by mouth or by injection into a muscle or into a vein.

Lapatinib (INN), used in the form of lapatinib ditosylate (USAN) is an orally active drug for breast cancer and other solid tumours. It is a dual tyrosine kinase inhibitor which interrupts the HER2/neu and epidermal growth factor receptor (EGFR) pathways. It is used in combination therapy for HER2-positive breast cancer. It is used for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress HER2 (ErbB2).
Neurokinin 1 (NK1) antagonists (-pitants) are a novel class of medications that possesses unique antidepressant, anxiolytic, and antiemetic properties. NK-1 antagonists boost the efficacy of 5-HT3 antagonists to prevent nausea and vomiting. The discovery of neurokinin 1 (NK1) receptor antagonists was a turning point in the prevention of nausea and vomiting associated with cancer chemotherapy.
Mapatumumab (HGS-ETR1) is an experimental human monoclonal antibody undergoing clinical trials for the treatment of cancer. It targets TRAIL-R1, also known as DR4, which is expressed on the surface of many tumor cell types.

The 5-HT3 antagonists, informally known as "setrons", are a class of drugs that act as receptor antagonists at the 5-HT3 receptor, a subtype of serotonin receptor found in terminals of the vagus nerve and in certain areas of the brain. With the notable exceptions of alosetron and cilansetron, which are used in the treatment of irritable bowel syndrome, all 5-HT3 antagonists are antiemetics, used in the prevention and treatment of nausea and vomiting. They are particularly effective in controlling the nausea and vomiting produced by cancer chemotherapy and are considered the gold standard for this purpose.

Elesclomol is a drug that triggers apoptosis in cancer cells. It is being developed by Synta Pharmaceuticals and GlaxoSmithKline as a chemotherapy adjuvant, and has received both fast track and orphan drug status from the U.S. Food and Drug Administration for the treatment of metastatic melanoma. Synta Pharmaceuticals announced on February 26, 2009 the suspension of all clinical trials involving Elesclomol due to safety concerns. In March 2010, Synta announced that the FDA had approved resuming clinical development of elesclomol, and that they expected to initiate one or more clinical trials for elesclomol in the second half of the year.

Retigabine (INN) or ezogabine (USAN) is an anticonvulsant used as an adjunctive treatment for partial epilepsies in treatment-experienced adult patients. The drug was developed by Valeant Pharmaceuticals and GlaxoSmithKline. It was approved by the European Medicines Agency under the trade name Trobalt on March 28, 2011, and by the United States Food and Drug Administration (FDA), under the trade name Potiga, on June 10, 2011. Production was discontinued in June 2017.
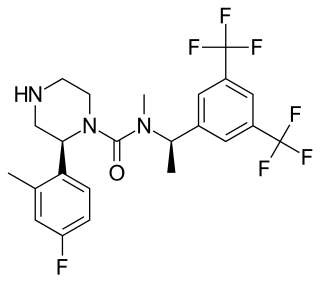
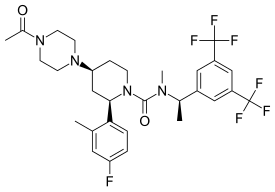
Vestipitant (INN) is a drug developed by GlaxoSmithKline which acts as a selective antagonist for the NK1 receptor. It is under development as a potential antiemetic and anxiolytic drug, and as a treatment for tinnitus and insomnia.

NS-2359 (GSK-372,475) is a serotonin-norepinephrine-dopamine reuptake inhibitor. It was under development by GlaxoSmithKline (GSK) as an antidepressant, but was discontinued in 2009 when phase II clinical trials showed the drug was not effective and not well tolerated. The results did not support further effort by the company. NS-2359 was also in clinical trials for the treatment of ADHD, phase II having been completed in 2007. A phase I clinical trial exploring the effect of NS-2359 on cocaine-dependent individuals was completed in 2002.
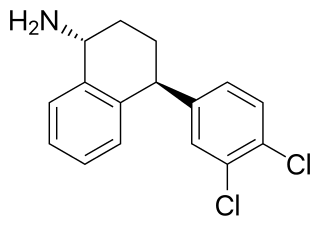
Dasotraline is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) that was under development by Sunovion for the treatment of attention-deficit hyperactivity disorder (ADHD) and binge eating disorder (BED). Structurally, dasotraline is a stereoisomer of desmethylsertraline (DMS), which is an active metabolite of the marketed selective serotonin reuptake inhibitor (SSRI) antidepressant sertraline (Zoloft).
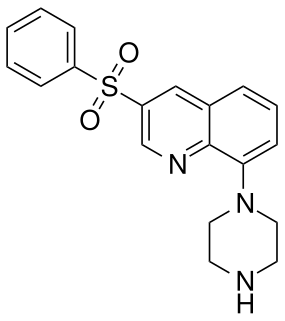
Intepirdine (INN; developmental codes SB-742457, RVT-101) is a selective 5-HT6 receptor antagonist with potential cognition, memory, and learning-enhancing effects. It was under development by GlaxoSmithKline for the treatment of Alzheimer's disease and demonstrated some preliminary efficacy in phase II clinical trials. GSK chose not to continue development and sold the rights to Axovant Sciences for $5 million in December 2014.
Chemotherapy-induced nausea and vomiting (CINV) is a common side-effect of many cancer treatments. Nausea and vomiting are two of the most feared cancer treatment-related side effects for cancer patients and their families. In 1983, Coates et al. found that patients receiving chemotherapy ranked nausea and vomiting as the first and second most severe side effects, respectively. Up to 20% of patients receiving highly emetogenic agents in this era postponed, or even refused, potentially curative treatments. Since the 1990s, several novel classes of antiemetics have been developed and commercialized, becoming a nearly universal standard in chemotherapy regimens, and helping to better manage these symptoms in a large portion of patients. Efficient mediation of these unpleasant and sometimes crippling symptoms results in increased quality of life for the patient, and better overall health of the patient, and, due to better patient tolerance, more effective treatment cycles.
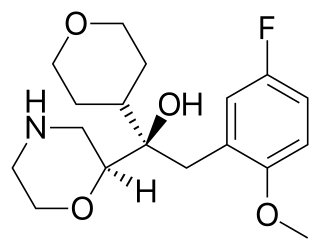
Edivoxetine is a drug which acts as a selective norepinephrine reuptake inhibitor and was under development by Eli Lilly for attention-deficit disorder (ADD) and as an antidepressant treatment. It was in phase III clinical trials, in 2012, for major depressive disorder, but failed to get approval.

Cancer and nausea are associated in about fifty percent of people affected by cancer. This may be as a result of the cancer itself, or as an effect of the treatment such as chemotherapy, radiation therapy, or other medication such as opiates used for pain relief. About 70 to 80% of people undergoing chemotherapy experience nausea or vomiting. Nausea and vomiting may also occur in people not receiving treatment, often as a result of the disease involving the gastrointestinal tract, electrolyte imbalance, or as a result of anxiety. Nausea and vomiting may be experienced as the most unpleasant side effects of cytotoxic drugs and may result in patients delaying or refusing further radiotherapy or chemotherapy.
Sufotidine (INN, USAN, codenamed AH25352) is a long-acting competitive H2 receptor antagonist which was under development as an antiulcerant by Glaxo (now GlaxoSmithKline). It was planned to be a follow-up compound to ranitidine (Zantac). When taken in doses of 600 mg twice daily it induced virtually 24-hour gastric anacidity thus closely resembling the antisecretory effect of the proton pump inhibitor omeprazole. Its development was terminated in 1989 from phase III clinical trials based on the appearance of carcinoid tumors in long-term toxicity testing in rodents.

Rolapitant (INN, trade name Varubivə-ROO-bee in the US and Varuby in the European Union) is a drug originally developed by Schering-Plough and licensed for clinical development by Tesaro, which acts as a selective NK1 receptor antagonist (antagonist for the NK1 receptor). It has been approved as a medication for the treatment of chemotherapy-induced nausea and vomiting (CINV) after clinical trials showed it to have similar or improved efficacy and some improvement in safety over existing drugs for this application.
Dostarlimab, sold under the brand name Jemperli, is a monoclonal antibody used as a medication for the treatment of endometrial cancer. Dostarlimab is a programmed death receptor-1 (PD-1)–blocking monoclonal antibody.
Belantamab mafodotin, sold under the brand name Blenrep, is a medication for the treatment of relapsed and refractory multiple myeloma.