
Tachykinin peptides are one of the largest families of neuropeptides, found from amphibians to mammals. They were so named due to their ability to rapidly induce contraction of gut tissue. The tachykinin family is characterized by a common C-terminal sequence, Phe-X-Gly-Leu-Met-NH2, where X is either an Aromatic or an Aliphatic amino acid. The genes that produce tachykinins encode precursor proteins called preprotachykinins, which are chopped apart into smaller peptides by posttranslational proteolytic processing. The genes also code for multiple splice forms that are made up of different sets of peptides.
Neurokinin 1 (NK1) antagonists (-pitants) are a novel class of medications that possesses unique antidepressant, anxiolytic, and antiemetic properties. NK-1 antagonists boost the efficacy of 5-HT3 antagonists to prevent nausea and vomiting. The discovery of neurokinin 1 (NK1) receptor antagonists was a turning point in the prevention of nausea and vomiting associated with cancer chemotherapy.

Neurokinin A (NKA), formerly known as Substance K, is a neurologically active peptide translated from the pre-protachykinin gene. Neurokinin A has many excitatory effects on mammalian nervous systems and is also influential on the mammalian inflammatory and pain responses.
The tachykinin receptor 1 (TACR1) also known as neurokinin 1 receptor (NK1R) or substance P receptor (SPR) is a G protein coupled receptor found in the central nervous system and peripheral nervous system. The endogenous ligand for this receptor is Substance P, although it has some affinity for other tachykinins. The protein is the product of the TACR1 gene.

Substance-K receptor is a protein that in humans is encoded by the TACR2 gene.

Tachykinin receptor 3, also known as TACR3, is a protein which in humans is encoded by the TACR3 gene.
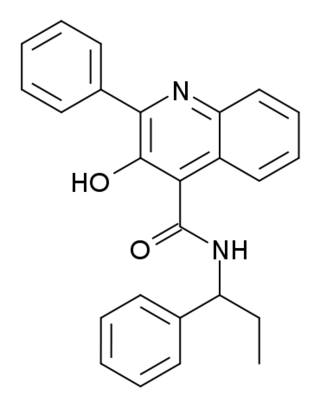
Talnetant (SB-223,412) is a neurokinin 3 receptor antagonist developed by GlaxoSmithKline, which is being researched for several functions. Its use as a potential antipsychotic drug for the treatment of schizophrenia has also been discontinued.

Osanetant (developmental code name SR-142,801) is a neurokinin 3 receptor antagonist which was developed by Sanofi-Synthélabo and was being researched for the treatment of schizophrenia but was discontinued. It was the first non-peptide NK3 antagonist developed in the mid-1990s.
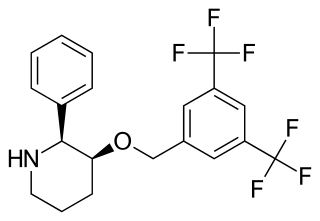
L-733,060 is a drug developed by Merck which acts as an orally active, non-peptide, selective antagonist for the NK1 receptor, binding with a Ki of 0.08 nM. Only one enantiomer is active which has made it the subject of several asymmetric synthesis efforts.

Ecopipam is a dopamine antagonist which is under development for the treatment of Lesch-Nyhan syndrome, Tourette's syndrome, speech disorders, and restless legs syndrome. It is taken by mouth.

Samidorphan is an opioid antagonist that in the form of olanzapine/samidorphan is used in the treatment of schizophrenia and bipolar disorder. Samidorphan reduces the weight gain associated with olanzapine. Samidorphan is taken by mouth.

Vofopitant (GR205171) is a drug which acts as an NK1 receptor antagonist. It has antiemetic effects as with other NK1 antagonists, and also shows anxiolytic actions in animals. It was studied for applications such as the treatment of social phobia and post-traumatic stress disorder, but did not prove sufficiently effective to be marketed.
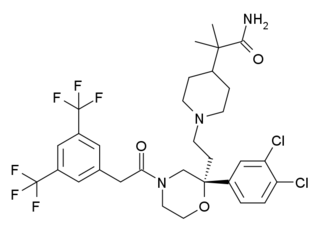
Burapitant (SSR-240,600) is a drug developed by Sanofi-Aventis which was one of the first compounds developed that acts as a potent and selective antagonist for the NK1 receptor. While burapitant itself did not proceed beyond early clinical trials and was never developed for clinical use in humans, promising animal results from this and related compounds have led to a number of novel drugs from this class that have now been introduced into medical use.

Fezolinetant, sold under the brand name Veozah among others, is a medication used for the treatment of hot flashes (vasomotor symptoms) due to menopause. It is a small-molecule, orally active, selective neurokinin-3 (NK3) receptor antagonist which is under development by for the treatment of sex hormone-related disorders. It is developed by Astellas Pharma which acquired it from Ogeda (formerly Euroscreen) in April 2017.

Amesergide is a serotonin receptor antagonist of the ergoline and lysergamide families related to methysergide which was under development by Eli Lilly and Company for the treatment of a variety of conditions including depression, anxiety, schizophrenia, male sexual dysfunction, migraine, and thrombosis but was never marketed. It reached phase II clinical trials for the treatment of depression, erectile dysfunction, and premature ejaculation prior to the discontinuation of its development.
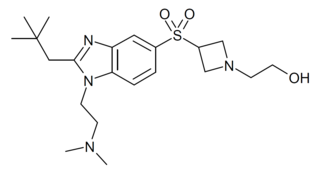
RQ-00202730 is a benzimidazole derived drug that acts as a potent and highly selective agonist for the CB2 cannabinoid receptor, with a Ki value of 19nM at CB2 and more than 4000x selectivity over CB1, though it also shows some activity as an antagonist of the unrelated 5-HT2B serotonin receptor. It has analgesic and antiinflammatory effects in animal studies, and was developed for the treatment of irritable bowel syndrome, but was ultimately discontinued from development following disappointing results in Phase II clinical trials.

Elinzanetant (developmental code names BAY-3427080GSK-1144814, NT-814) is an orally active small-molecule neurokinin/tachykinin NK1 receptor and NK3 receptor antagonist which is under development by Bayer, GlaxoSmithKline, and NeRRe Therapeutics for the treatment of hot flashes and "sex hormone disorders". It has been found to relieve hot flashes in postmenopausal women and to dose-dependently suppress luteinizing hormone, estradiol, and progesterone levels in premenopausal women. As of August 2021, elinzanetant is in phase 2 clinical trials for hot flashes and "sex hormone disorders". It was also under development for the treatment of schizophrenia and opioid-related disorders, but development was discontinued for these uses.

Trazpiroben (developmental code name TAK-906) is a dopamine antagonist drug which was under development for the treatment of gastroparesis. It acts as a peripherally selective dopamine D2 and D3 receptor antagonist. The drug has been found to strongly increase prolactin levels in humans, similarly to other peripherally selective D2 receptor antagonists like domperidone. Clinical development of trazpiroben was discontinued before April 2022. Trazpiroben was originated by Altos Therapeutics and was under development by Takeda Oncology.

















