Angiotensin-converting-enzyme inhibitors are a class of medication used primarily for the treatment of high blood pressure and heart failure. This class of medicine works by causing relaxation of blood vessels as well as a decrease in blood volume, which leads to lower blood pressure and decreased oxygen demand from the heart.
Antihypertensives are a class of drugs that are used to treat hypertension. Antihypertensive therapy seeks to prevent the complications of high blood pressure, such as stroke, heart failure, kidney failure and myocardial infarction. Evidence suggests that reduction of the blood pressure by 5 mmHg can decrease the risk of stroke by 34% and of ischaemic heart disease by 21%, and can reduce the likelihood of dementia, heart failure, and mortality from cardiovascular disease. There are many classes of antihypertensives, which lower blood pressure by different means. Among the most important and most widely used medications are thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors (ACEis), angiotensin II receptor blockers or antagonists (ARBs), and beta blockers.

Enalapril, sold under the brand name Vasotec among others, is an ACE inhibitor medication used to treat high blood pressure, diabetic kidney disease, and heart failure. For heart failure, it is generally used with a diuretic, such as furosemide. It is given by mouth or by injection into a vein. Onset of effects are typically within an hour when taken by mouth and last for up to a day.

Angiotensin II receptor blockers (ARBs), formally angiotensin II receptor type 1 (AT1) antagonists, also known as angiotensin receptor blockers, angiotensin II receptor antagonists, or AT1 receptor antagonists, are a group of pharmaceuticals that bind to and inhibit the angiotensin II receptor type 1 (AT1) and thereby block the arteriolar contraction and sodium retention effects of renin–angiotensin system.
Candesartan is an angiotensin receptor blocker used mainly for the treatment of high blood pressure and congestive heart failure. Candesartan has a very low maintenance dose. Like olmesartan, the metabolism of the drug is unusual as it is a cascading prodrug. Candesartan has good bioavailibility and is the most potent by weight of the AT-1 receptor antagonists.

Diabetic nephropathy, also known as diabetic kidney disease, is the chronic loss of kidney function occurring in those with diabetes mellitus. Diabetic nephropathy is the leading causes of chronic kidney disease (CKD) and end-stage renal disease (ESRD) globally. The triad of protein leaking into the urine, rising blood pressure with hypertension and then falling renal function is common to many forms of CKD. Protein loss in the urine due to damage of the glomeruli may become massive, and cause a low serum albumin with resulting generalized body swelling (edema) so called nephrotic syndrome. Likewise, the estimated glomerular filtration rate (eGFR) may progressively fall from a normal of over 90 ml/min/1.73m2 to less than 15, at which point the patient is said to have end-stage renal disease. It usually is slowly progressive over years.

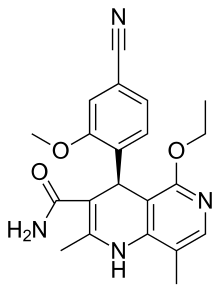
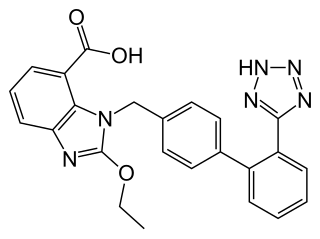
Telmisartan, sold under the brand name Micardis among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth. Versions are available as the combination telmisartan/hydrochlorothiazide, telmisartan/cilnidipine and telmisartan/amlodipine.

Potassium-sparing diuretics or antikaliuretics refer to drugs that cause diuresis without causing potassium loss in the urine. They are typically used as an adjunct in management of hypertension, cirrhosis, and congestive heart failure. The steroidal aldosterone antagonists can also be used for treatment of primary hyperaldosteronism. Spironolactone, a steroidal aldosterone antagonist, is also used in management of female hirsutism and acne from PCOS or other causes.
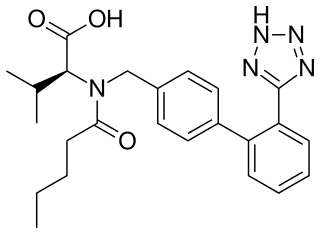
Valsartan, sold under the brand name Diovan among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It belongs to a class of medications referred to as angiotensin II receptor blockers (ARBs). It is a reasonable initial treatment for high blood pressure. It is taken by mouth.

Fenofibrate (sold under the brand name Tricor among others, is an oral medication of the fibrate class used to treat abnormal blood lipid levels. It is less commonly used compared than statins because it treats a different type of cholesterol abnormality to statins. While statins have strong evidence for reducing heart disease and death, there is evidence to suggest that fenofibrate also reduces the risk of heart disease and death. However, this seems only to apply to specific populations of people with elevated triglyceride levels and reduced high-density lipoprotein cholesterol. Its use is recommended together with dietary changes.
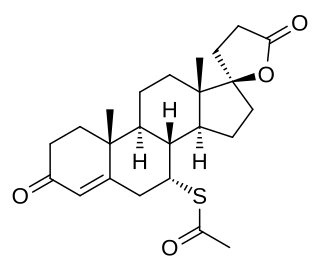
A mineralocorticoid receptor antagonist or aldosterone antagonist, is a diuretic drug which antagonizes the action of aldosterone at mineralocorticoid receptors. This group of drugs is often used as adjunctive therapy, in combination with other drugs, for the management of chronic heart failure. Spironolactone, the first member of the class, is also used in the management of hyperaldosteronism and female hirsutism. Most antimineralocorticoids, including spironolactone, are steroidal spirolactones. Finerenone is a nonsteroidal antimineralocorticoid.

Eplerenone, sold under the brand name Inspra, is an aldosterone antagonist type of potassium-sparing diuretic that is used to treat chronic heart failure and high blood pressure, particularly for people with resistant hypertension due to elevated aldosterone. It is a steroidal antimineralocorticoid of the spirolactone group and a selective aldosterone receptor antagonist (SARA).

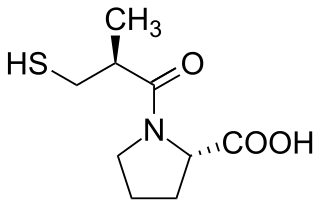
Perindopril is a medication used to treat high blood pressure, heart failure, or stable coronary artery disease. As a long-acting ACE inhibitor, it works by relaxing blood vessels and decreasing blood volume. As a prodrug, perindopril is hydrolyzed in the liver to its active metabolite, perindoprilat. It was patented in 1980 and approved for medical use in 1988.
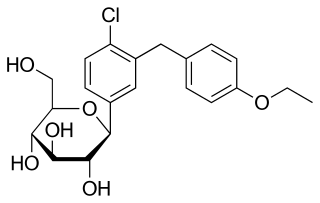
Dapagliflozin, sold under the brand names Farxiga (US) and Forxiga (EU) among others, is a medication used to treat type 2 diabetes. It is also used to treat adults with heart failure and chronic kidney disease. It reversibly inhibits sodium-glucose co-transporter 2 (SGLT-2) in the renal proximal convoluted tubule to reduce glucose reabsorption and increase urinary glucose excretion.

Atrasentan is an experimental drug that is being studied for the treatment of various types of cancer, including non-small cell lung cancer. It is also being investigated as a therapy for diabetic kidney disease.

Canagliflozin, sold under the brand name Invokana among others, is a medication used to treat type 2 diabetes. It is used together with exercise and diet. It is not recommended in type 1 diabetes. It is taken by mouth.
Empagliflozin, sold under the brand name Jardiance, among others, is an antidiabetic medication used to improve glucose control in people with type 2 diabetes. It is taken by mouth.

Dulaglutide, sold under the brand name Trulicity among others, is a medication used for the treatment of type 2 diabetes in combination with diet and exercise. It is also approved in the United States for the reduction of major adverse cardiovascular events in adults with type 2 diabetes who have established cardiovascular disease or multiple cardiovascular risk factors.
SGLT2 inhibitors are a class of medications that inhibit sodium-glucose transport proteins in the nephron, unlike SGLT1 inhibitors that perform a similar function in the intestinal mucosa. The foremost metabolic effect of this is to inhibit reabsorption of glucose in the kidney and therefore lower blood sugar. They act by inhibiting sodium/glucose cotransporter 2 (SGLT2). SGLT2 inhibitors are used in the treatment of type 2 diabetes. Apart from blood sugar control, gliflozins have been shown to provide significant cardiovascular benefit in people with type 2 diabetes. As of 2014, several medications of this class had been approved or were under development. In studies on canagliflozin, a member of this class, the medication was found to enhance blood sugar control as well as reduce body weight and systolic and diastolic blood pressure.
Sotagliflozin, sold under the brand name Inpefa among others, is a medication used to reduce the risk of death due to heart failure. It is a sodium-glucose cotransporter 2 (SGLT2) inhibitor. It is taken by mouth.